当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chain transfer to solvent in BN 2-vinylnaphthalene radical polymerization
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2021-06-21 , DOI: 10.1002/pol.20210362 Yuyang Ji 1 , Rebekka S. Klausen 1
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2021-06-21 , DOI: 10.1002/pol.20210362 Yuyang Ji 1 , Rebekka S. Klausen 1
Affiliation
|
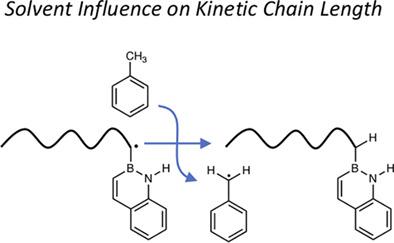
The synthesis of vinyl alcohol copolymers is limited due to the poor radical reactivity of vinyl acetate (VAc), the traditional precursor to polyvinyl alcohol (PVA). Main group monomers such as BN 2-vinylnaphthalene (BN2VN) have attracted attention as alternatives to VAc to form side chain hydroxyls via oxidation, but outstanding questions of molecular weight control remain. Herein we report systematic investigation of solvent, temperature, and initiator concentration as factors influencing BN2VN degree of polymerization. We find increased chain transfer to toluene, hypothesized to arise from differences in radical stabilization and reactivity by aromatic and BN aromatic rings. As a result of these combined efforts, high molecular weight (Mw ~ 105 g mol−1) BN2VN homopolymers and BN2VN-styrene copolymers were obtained.
中文翻译:

BN 2-乙烯基萘自由基聚合中的链转移到溶剂
由于醋酸乙烯酯 (VAc) 是聚乙烯醇 (PVA) 的传统前体,其自由基反应性较差,因此乙烯醇共聚物的合成受到限制。BN 2-乙烯基萘 (BN2VN) 等主要单体作为 VAc 的替代品通过氧化形成侧链羟基已引起关注,但分子量控制的突出问题仍然存在。在此,我们报告了溶剂、温度和引发剂浓度作为影响 BN2VN 聚合度的因素的系统研究。我们发现向甲苯的链转移增加,推测是由于芳环和 BN 芳环在自由基稳定性和反应性方面的差异引起的。作为这些共同努力的结果,高分子量 (M w ~ 10 5 g mol -1)得到BN2VN均聚物和BN2VN-苯乙烯共聚物。
更新日期:2021-06-21
中文翻译:

BN 2-乙烯基萘自由基聚合中的链转移到溶剂
由于醋酸乙烯酯 (VAc) 是聚乙烯醇 (PVA) 的传统前体,其自由基反应性较差,因此乙烯醇共聚物的合成受到限制。BN 2-乙烯基萘 (BN2VN) 等主要单体作为 VAc 的替代品通过氧化形成侧链羟基已引起关注,但分子量控制的突出问题仍然存在。在此,我们报告了溶剂、温度和引发剂浓度作为影响 BN2VN 聚合度的因素的系统研究。我们发现向甲苯的链转移增加,推测是由于芳环和 BN 芳环在自由基稳定性和反应性方面的差异引起的。作为这些共同努力的结果,高分子量 (M w ~ 10 5 g mol -1)得到BN2VN均聚物和BN2VN-苯乙烯共聚物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号