当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed asymmetric cyclization of alkenyl diynes: method development and new mechanistic insights
Chemical Science ( IF 7.6 ) Pub Date : 2021-6-11 , DOI: 10.1039/d1sc02773e
Xin-Qi Zhu 1 , Pan Hong 1 , Yan-Xin Zheng 1 , Ying-Ying Zhen 1 , Feng-Lin Hong 1 , Xin Lu 1 , Long-Wu Ye 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2021-6-11 , DOI: 10.1039/d1sc02773e
Xin-Qi Zhu 1 , Pan Hong 1 , Yan-Xin Zheng 1 , Ying-Ying Zhen 1 , Feng-Lin Hong 1 , Xin Lu 1 , Long-Wu Ye 1, 2
Affiliation
|
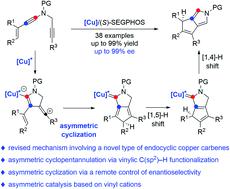
Metal carbenes have proven to be one of the most important and useful intermediates in organic synthesis, but catalytic asymmetric reactions involving metal carbenes are still scarce and remain a challenge. Particularly, the mechanistic pathway and chiral induction model in these asymmetric transformations are far from clear. Described herein is a copper-catalyzed asymmetric cyclization of alkenyl diynes involving a vinylic C(sp2)–H functionalization, which constitutes the first asymmetric vinylic C(sp2)–H functionalization through cyclopentannulation. Significantly, based on extensive mechanistic studies including control experiments and theoretical calculations, a revised mechanism involving a novel type of endocyclic copper carbene via remote-stereocontrol is proposed, thus providing new mechanistic insight into the copper-catalyzed asymmetric diyne cyclization and representing a new chiral control pattern in asymmetric catalysis based on remote-stereocontrol and vinyl cations. This method enables the practical and atom-economical construction of an array of valuable chiral polycyclic-pyrroles in high yields and enantioselectivities.
中文翻译:

铜催化烯基二炔的不对称环化:方法开发和新的机理见解
金属卡宾已被证明是有机合成中最重要和最有用的中间体之一,但涉及金属卡宾的催化不对称反应仍然很少,并且仍然是一个挑战。特别是,这些不对称转化中的机制途径和手性诱导模型还远未明确。本文描述的是铜催化的烯基二炔不对称环化,涉及乙烯基 C(sp 2 )-H 官能化,它构成了第一个通过环戊环化作用的不对称乙烯基 C(sp 2 )-H 官能化。值得注意的是,基于广泛的机理研究,包括控制实验和理论计算,一种涉及新型内环铜卡宾的修正机制通过提出了远程立体控制,从而为铜催化的不对称二炔环化提供了新的机理见解,并代表了基于远程立体控制和乙烯基阳离子的不对称催化中的新手性控制模式。该方法能够以高产率和对映选择性构建一系列有价值的手性多环吡咯的实用且原子经济的结构。
更新日期:2021-06-21
中文翻译:

铜催化烯基二炔的不对称环化:方法开发和新的机理见解
金属卡宾已被证明是有机合成中最重要和最有用的中间体之一,但涉及金属卡宾的催化不对称反应仍然很少,并且仍然是一个挑战。特别是,这些不对称转化中的机制途径和手性诱导模型还远未明确。本文描述的是铜催化的烯基二炔不对称环化,涉及乙烯基 C(sp 2 )-H 官能化,它构成了第一个通过环戊环化作用的不对称乙烯基 C(sp 2 )-H 官能化。值得注意的是,基于广泛的机理研究,包括控制实验和理论计算,一种涉及新型内环铜卡宾的修正机制通过提出了远程立体控制,从而为铜催化的不对称二炔环化提供了新的机理见解,并代表了基于远程立体控制和乙烯基阳离子的不对称催化中的新手性控制模式。该方法能够以高产率和对映选择性构建一系列有价值的手性多环吡咯的实用且原子经济的结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号