当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular difunctionalization of methylenecyclopropanes tethered with carboxylic acid by visible-light photoredox catalysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-6-15 , DOI: 10.1039/d1qo00564b
Hao-Zhao Wei 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-6-15 , DOI: 10.1039/d1qo00564b
Hao-Zhao Wei 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Affiliation
|
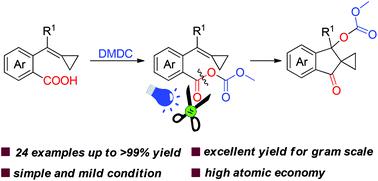
We have developed a visible-light photoredox catalyzed intramolecular difunctionalization of MCPs to access spiro[cyclopropane-1,2-indan]one from easily prepared methylenecyclopropanes tethered with carboxylic acid. The reaction can be performed under mild conditions, affording target products in excellent yields with a high atomic economy, and this cyclization reaction can be extended to gram scale synthesis. The products could be transformed to 1-indanone motifs existing in many natural products and bioactive compounds.
中文翻译:

通过可见光光氧化还原催化与羧酸相连的亚甲基环丙烷的分子内双官能化
我们开发了一种可见光光氧化还原催化的 MCP 分子内双官能化,以从易于制备的与羧酸相连的亚甲基环丙烷中获得螺[环丙烷-1,2-茚满]酮。该反应可以在温和的条件下进行,以高原子经济性以优异的收率提供目标产物,并且该环化反应可以扩展到克级合成。这些产物可以转化为存在于许多天然产物和生物活性化合物中的 1-茚满酮基序。
更新日期:2021-06-21
中文翻译:

通过可见光光氧化还原催化与羧酸相连的亚甲基环丙烷的分子内双官能化
我们开发了一种可见光光氧化还原催化的 MCP 分子内双官能化,以从易于制备的与羧酸相连的亚甲基环丙烷中获得螺[环丙烷-1,2-茚满]酮。该反应可以在温和的条件下进行,以高原子经济性以优异的收率提供目标产物,并且该环化反应可以扩展到克级合成。这些产物可以转化为存在于许多天然产物和生物活性化合物中的 1-茚满酮基序。































 京公网安备 11010802027423号
京公网安备 11010802027423号