当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
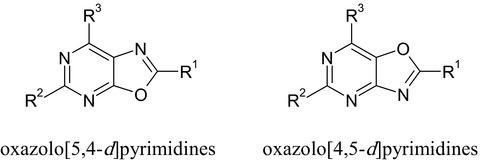
Intrinsic drug potential of oxazolo[5,4-d]pyrimidines and oxazolo[4,5-d]pyrimidines
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-06-20 , DOI: 10.1111/cbdd.13911 Victor V. Zhirnov 1 , Yevheniia S. Velihina 1 , Oleg P. Mitiukhin 1 , Volodymyr S. Brovarets 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-06-20 , DOI: 10.1111/cbdd.13911 Victor V. Zhirnov 1 , Yevheniia S. Velihina 1 , Oleg P. Mitiukhin 1 , Volodymyr S. Brovarets 1
Affiliation
|
The oxazole and pyrimidine rings are widely displayed in natural products and synthetic molecules. They are known as the prime skeletons for drug discovery. On the account of structural and chemical diversity, oxazole and pyrimidine-based molecules, as central scaffolds, not only provide different types of interactions with various receptors and enzymes, showing broad biological activities, but also occupy a core position in medicinal chemistry, showing their importance for development and discovery of newer potential therapeutic agents (Curr Top Med Chem, 16, 2016, 3133; Int J Pharm Pharm Sci, 8, 2016, 8; BMC Chem, 13, 2019, 44). For a long time, relatively little attention has been paid to their fused rings that are oxazolopyrimidines, whose chemical structure is similar to that of natural purines because probably none of these compounds were found in natural products or their biological activities turned out to be unexpressed (Bull Chem Soc Jpn, 43, 1970, 187). Recently, however, a significant number of studies have been published on the biological properties of oxazolo[5,4-d]pyrimidines, showing their significant activity as agonists and antagonists of signaling pathways involved in the regulation of the cell life cycle, whereas oxazolo[4,5-d]pyrimidines, on the contrary, represent a poorly studied class of compounds. Limited access to this scaffold has resulted in a corresponding lack of biological research (Eur J Organ Chem, 18, 2018, 2148). Actually, oxazolo[5,4-d]pyrimidine is a versatile scaffold used for the design of bioactive ligands against enzymes and receptors. This review focuses on biological targets and associated pathogenetic mechanisms, as well as pathological disorders that can be modified by well-known oxazolopyrimidines that have been proven to date. Many molecular details of these processes are omitted here, which the interested reader will find in the cited literature. This work also does not cover the methods for the synthesis of the oxazolopyrimidines, which are exhaustively described by De Coen et al. (Eur J Organ Chem, 18, 2018, 2148). The review as well does not discuss the structure–activity relationship, which is described in detail in the original works and deliberately, whenever possible, cites not primary sources, but mostly relevant review articles, so that the reader who wants to delve into a particular problem will immediately receive more complete information. It is expected that the information presented in this review will help readers better understand the purpose of the development of oxazolopyrimidines and the possibility of their development as drugs for the treatment of a wide range of diseases.
中文翻译:

恶唑并[5,4-d]嘧啶和恶唑并[4,5-d]嘧啶的内在药物潜力
恶唑和嘧啶环广泛存在于天然产物和合成分子中。它们被称为药物发现的主要骨架。由于结构和化学的多样性,恶唑和嘧啶类分子作为中心支架,不仅提供与各种受体和酶的不同类型的相互作用,显示出广泛的生物活性,而且在药物化学中占据核心地位,显示出它们的作用。开发和发现新的潜在治疗药物的重要性 ( Curr Top Med Chem , 16 , 2016, 3133; Int J Pharm Pharm Sci , 8 , 2016, 8; BMC Chem , 13, 2019, 44)。长期以来,它们的稠环是恶唑并嘧啶,其化学结构与天然嘌呤相似,因此很少受到关注,因为这些化合物可能都没有在天然产物中发现,或者它们的生物活性被证明是未表达的。Bull Chem Soc Jpn , 43 , 1970, 187)。然而,最近发表了大量关于恶唑并[5,4 -d ]嘧啶的生物学特性的研究,表明它们作为参与细胞生命周期调节的信号通路的激动剂和拮抗剂具有显着的活性,而恶唑并[4,5 -d]嘧啶,相反,代表了一类研究不足的化合物。对该支架的有限访问导致相应缺乏生物学研究(Eur J Organ Chem , 18 , 2018, 2148)。实际上,恶唑并[5,4 -d]嘧啶是一种多功能支架,用于设计针对酶和受体的生物活性配体。本综述重点关注生物学靶点和相关的发病机制,以及可以通过迄今为止已被证明的众所周知的恶唑并嘧啶修饰的病理障碍。这里省略了这些过程的许多分子细节,感兴趣的读者可以在引用的文献中找到。这项工作也不包括 De Coen 等人详尽描述的恶唑并嘧啶的合成方法。(欧洲器官化学杂志, 18, 2018, 2148)。综述也没有讨论结构-活性关系,这在原著中有详细描述,并有意尽可能不引用主要来源,而是大部分相关的综述文章,以便想要深入研究特定领域的读者问题将立即收到更完整的信息。预计本综述中提供的信息将有助于读者更好地了解开发恶唑并嘧啶类药物的目的以及将其开发为治疗多种疾病的药物的可能性。
更新日期:2021-06-20
中文翻译:

恶唑并[5,4-d]嘧啶和恶唑并[4,5-d]嘧啶的内在药物潜力
恶唑和嘧啶环广泛存在于天然产物和合成分子中。它们被称为药物发现的主要骨架。由于结构和化学的多样性,恶唑和嘧啶类分子作为中心支架,不仅提供与各种受体和酶的不同类型的相互作用,显示出广泛的生物活性,而且在药物化学中占据核心地位,显示出它们的作用。开发和发现新的潜在治疗药物的重要性 ( Curr Top Med Chem , 16 , 2016, 3133; Int J Pharm Pharm Sci , 8 , 2016, 8; BMC Chem , 13, 2019, 44)。长期以来,它们的稠环是恶唑并嘧啶,其化学结构与天然嘌呤相似,因此很少受到关注,因为这些化合物可能都没有在天然产物中发现,或者它们的生物活性被证明是未表达的。Bull Chem Soc Jpn , 43 , 1970, 187)。然而,最近发表了大量关于恶唑并[5,4 -d ]嘧啶的生物学特性的研究,表明它们作为参与细胞生命周期调节的信号通路的激动剂和拮抗剂具有显着的活性,而恶唑并[4,5 -d]嘧啶,相反,代表了一类研究不足的化合物。对该支架的有限访问导致相应缺乏生物学研究(Eur J Organ Chem , 18 , 2018, 2148)。实际上,恶唑并[5,4 -d]嘧啶是一种多功能支架,用于设计针对酶和受体的生物活性配体。本综述重点关注生物学靶点和相关的发病机制,以及可以通过迄今为止已被证明的众所周知的恶唑并嘧啶修饰的病理障碍。这里省略了这些过程的许多分子细节,感兴趣的读者可以在引用的文献中找到。这项工作也不包括 De Coen 等人详尽描述的恶唑并嘧啶的合成方法。(欧洲器官化学杂志, 18, 2018, 2148)。综述也没有讨论结构-活性关系,这在原著中有详细描述,并有意尽可能不引用主要来源,而是大部分相关的综述文章,以便想要深入研究特定领域的读者问题将立即收到更完整的信息。预计本综述中提供的信息将有助于读者更好地了解开发恶唑并嘧啶类药物的目的以及将其开发为治疗多种疾病的药物的可能性。








































 京公网安备 11010802027423号
京公网安备 11010802027423号