当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Kinetic Resolution of Carboxylic Esters Catalyzed by Chiral PPY N-Oxides: Synthesis of Nonsteroidal Anti-Inflammatory Drugs and Mechanistic Insights
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-18 , DOI: 10.1021/acscatal.1c01438
Ming-Sheng Xie 1 , Ning Li 1 , Yin Tian 2 , Xiao-Xia Wu 1 , Yun Deng 2 , Gui-Rong Qu 1 , Hai-Ming Guo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-18 , DOI: 10.1021/acscatal.1c01438
Ming-Sheng Xie 1 , Ning Li 1 , Yin Tian 2 , Xiao-Xia Wu 1 , Yun Deng 2 , Gui-Rong Qu 1 , Hai-Ming Guo 1
Affiliation
|
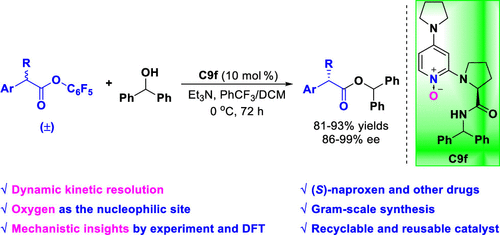
Bifunctional chiral 4-pyrrolidinopyridine (PPY) N-oxides catalyzing the dynamic kinetic resolution of racemic carboxylic esters was reported to construct chiral α-aryl α-alkyl carboxylic esters in up to 93% yield and 99% ee. Several esters of nonsteroidal anti-inflammatory drugs (NSAIDs), including (S)-ibuprofen ester, (S)-ketoprofen ester, (S)-fenaprofen ester, and (S)-flurbinprofen ester, were obtained. Additionally, the drug molecule (S)-naproxen could be achieved by hydrogenation of (S)-naproxen ester. The (S)-naproxen ester was prepared on a gram scale in 87% yield and 94% ee. The catalyst was recyclable and reusable. Mechanistic studies were conducted by control experiments, HRMS analysis, 1H NMR spectral detection, stereochemical experiments, racemization studies, competitive experiments, a linearity relationship determination, a kinetic order analysis, and a DFT computational study. The obtained results suggested that in PPY N-oxides the oxygen atom served as the nucleophilic site and N–H bond acted as the H-bond donor, resulting in a synergistic effect. In comparison with previous works using nitrogen or carbon as the nucleophilic sites, we found that chiral PPY N-oxides with oxygen as the nucleophilic site could also catalyze such reactions.
中文翻译:

手性 PPY N -氧化物催化羧酸酯的动态动力学拆分:非甾体抗炎药的合成及机理研究
据报道,双功能手性 4-吡咯烷并吡啶 (PPY) N-氧化物催化外消旋羧酸酯的动态动力学拆分,以高达 93% 的产率和 99% 的 ee 构建手性 α-芳基 α-烷基羧酸酯。获得了几种非甾体抗炎药(NSAIDs)的酯,包括(S)-布洛芬酯、(S)-酮洛芬酯、(S)-非那洛芬酯和(S)-氟苯丙芬酯。此外,药物分子( S )-萘普生可以通过( S )-萘普生酯的氢化来实现。( S)-萘普生酯以克规模制备,产率为 87%,ee 为 94%。该催化剂是可回收和可重复使用的。通过对照实验、HRMS 分析、1 H NMR 光谱检测、立体化学实验、外消旋化研究、竞争性实验、线性关系测定、动力学顺序分析和 DFT 计算研究进行机械研究。所得结果表明,在 PPY N-氧化物中,氧原子充当亲核位点,N-H 键充当 H 键供体,从而产生协同效应。与之前使用氮或碳作为亲核位点的工作相比,我们发现以氧为亲核位点的手性 PPY N -氧化物也可以催化这种反应。
更新日期:2021-07-02
中文翻译:

手性 PPY N -氧化物催化羧酸酯的动态动力学拆分:非甾体抗炎药的合成及机理研究
据报道,双功能手性 4-吡咯烷并吡啶 (PPY) N-氧化物催化外消旋羧酸酯的动态动力学拆分,以高达 93% 的产率和 99% 的 ee 构建手性 α-芳基 α-烷基羧酸酯。获得了几种非甾体抗炎药(NSAIDs)的酯,包括(S)-布洛芬酯、(S)-酮洛芬酯、(S)-非那洛芬酯和(S)-氟苯丙芬酯。此外,药物分子( S )-萘普生可以通过( S )-萘普生酯的氢化来实现。( S)-萘普生酯以克规模制备,产率为 87%,ee 为 94%。该催化剂是可回收和可重复使用的。通过对照实验、HRMS 分析、1 H NMR 光谱检测、立体化学实验、外消旋化研究、竞争性实验、线性关系测定、动力学顺序分析和 DFT 计算研究进行机械研究。所得结果表明,在 PPY N-氧化物中,氧原子充当亲核位点,N-H 键充当 H 键供体,从而产生协同效应。与之前使用氮或碳作为亲核位点的工作相比,我们发现以氧为亲核位点的手性 PPY N -氧化物也可以催化这种反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号