当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Menaquinone Biosynthesis: The Mechanism of 5,8-Dihydroxy-2-naphthoate Synthase (MqnD)
Biochemistry ( IF 2.9 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.biochem.1c00257 Hannah R Manion-Sommerhalter 1 , Dmytro Fedoseyenko 1 , Sumedh Joshi 1 , Tadhg P Begley 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.biochem.1c00257 Hannah R Manion-Sommerhalter 1 , Dmytro Fedoseyenko 1 , Sumedh Joshi 1 , Tadhg P Begley 1
Affiliation
|
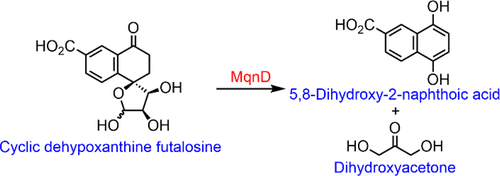
MqnD catalyzes the conversion of cyclic dehypoxanthine futalosine (6) to 5,8-dihydroxy-2-naphthoic acid (7) and an uncharacterized product. This study describes a chemoenzymatic synthesis of 6. This synthesis achieved a 2-fold yield enhancement by using titanium(III) citrate as the reducing agent and another 5-fold yield enhancement using a fluorinated analogue of dehypoxanthine futalosine (5) that was converted to 6 by an ipso substitution mechanism. This synthetic route enabled the synthesis of 6 in sufficient quantity to identify the second reaction product and to determine that the MqnD-catalyzed reaction proceeds by a hemiacetal ring opening–tautomerization–retroaldol sequence.
中文翻译:

甲基萘醌生物合成:5,8-二羟基-2-萘甲酸合酶 (MqnD) 的机制
MqnD 催化环状脱次黄嘌呤二氢呋喃 ( 6 ) 向 5,8-二羟基-2-萘甲酸 ( 7 ) 和未表征的产物的转化。本研究描述了6的化学酶促合成。通过使用柠檬酸钛 (III) 作为还原剂,该合成实现了 2 倍的产率提高,并使用通过 ipso取代机制转化为6的脱次黄嘌呤 futalosine ( 5 )的氟化类似物将产率提高了 5 倍。该合成路线能够合成足够数量的6以鉴定第二个反应产物,并确定 MqnD 催化的反应通过半缩醛开环-互变异构化-逆羟醛序列进行。
更新日期:2021-06-29
中文翻译:

甲基萘醌生物合成:5,8-二羟基-2-萘甲酸合酶 (MqnD) 的机制
MqnD 催化环状脱次黄嘌呤二氢呋喃 ( 6 ) 向 5,8-二羟基-2-萘甲酸 ( 7 ) 和未表征的产物的转化。本研究描述了6的化学酶促合成。通过使用柠檬酸钛 (III) 作为还原剂,该合成实现了 2 倍的产率提高,并使用通过 ipso取代机制转化为6的脱次黄嘌呤 futalosine ( 5 )的氟化类似物将产率提高了 5 倍。该合成路线能够合成足够数量的6以鉴定第二个反应产物,并确定 MqnD 催化的反应通过半缩醛开环-互变异构化-逆羟醛序列进行。






























 京公网安备 11010802027423号
京公网安备 11010802027423号