当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proximity-Induced Hybridization Chain Reaction-Based Photoacoustic Imaging System for Amplified Visualization Protein-Specific Glycosylation in Mice
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.analchem.1c01352 Zhenhua Liu 1 , Yuhua Liang 1 , Wenhua Cao 1 , Wen Gao 1 , Bo Tang 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.analchem.1c01352 Zhenhua Liu 1 , Yuhua Liang 1 , Wenhua Cao 1 , Wen Gao 1 , Bo Tang 1
Affiliation
|
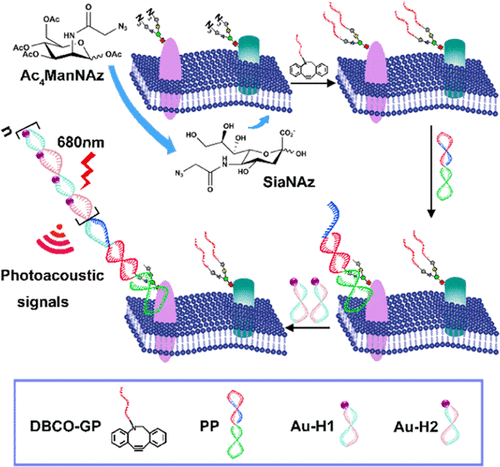
Glycosylation is a key cellular mechanism that regulates several physiological and pathological functions. Therefore, identification and characterization of specific-protein glycosylation in vivo are highly desirable for studying glycosylation-related pathology and developing personalized theranostic modalities. Herein, we demonstrated a photoacoustic (PA) nanoprobe based on the proximity-induced hybridization chain reaction (HCR) for amplified visual detection of protein-specific glycosylation in vivo. Two kinds of functional DNA probes were designed. A glycan probe (DBCO-GP) was attached to glycans through metabolic oligosaccharide engineering (MOE) and protein probe (PP)-targeted proteins by aptamer recognition. Proximity-induced hybridization of the complementary domain between the two kinds of probes promoted conformational changes in the protein probes and in situ release of the HCR initiator domain. Gold nanoparticles (AuNPs) modified by complementary sequences (Au-H1 and Au-H2) self-assembled into Au aggregates via the HCR, thereby converting DNA signals to photoacoustic signals. Due to the high contrast and deep penetration of photoacoustic imaging, this strategy enabled in situ detection of Mucin 1 (MUC1)-specific glycosylation in mice with breast cancer and successfully monitored its dynamic states during tunicamycin treatment. This imaging technique provides a powerful platform for studying the effects of glycosylation on the protein structure and function, which helps to elucidate its role in disease processes.
中文翻译:

用于放大可视化小鼠蛋白质特异性糖基化的基于邻近诱导杂交链反应的光声成像系统
糖基化是调节多种生理和病理功能的关键细胞机制。因此,体内特异性蛋白质糖基化的鉴定和表征对于研究糖基化相关的病理学和开发个性化的治疗诊断方法非常必要。在此,我们展示了一种基于邻近诱导杂交链反应 (HCR) 的光声 (PA) 纳米探针,用于体内蛋白质特异性糖基化的放大视觉检测。设计了两种功能性DNA探针。聚糖探针 (DBCO-GP) 通过代谢寡糖工程 (MOE) 和蛋白质探针 (PP) 靶向蛋白质通过适体识别连接到聚糖上。两种探针之间互补结构域的邻近诱导杂交促进了蛋白质探针的构象变化和 HCR 起始结构域的原位释放。由互补序列(Au-H1 和 Au-H2)修饰的金纳米粒子 (AuNPs) 通过 HCR 自组装成 Au 聚集体,从而将 DNA 信号转换为光声信号。由于光声成像的高对比度和深度穿透,该策略能够原位检测乳腺癌小鼠中的粘蛋白 1 (MUC1) 特异性糖基化,并在衣霉素治疗期间成功监测其动态状态。这种成像技术为研究糖基化对蛋白质结构和功能的影响提供了一个强大的平台,有助于阐明其在疾病过程中的作用。
更新日期:2021-06-29
中文翻译:

用于放大可视化小鼠蛋白质特异性糖基化的基于邻近诱导杂交链反应的光声成像系统
糖基化是调节多种生理和病理功能的关键细胞机制。因此,体内特异性蛋白质糖基化的鉴定和表征对于研究糖基化相关的病理学和开发个性化的治疗诊断方法非常必要。在此,我们展示了一种基于邻近诱导杂交链反应 (HCR) 的光声 (PA) 纳米探针,用于体内蛋白质特异性糖基化的放大视觉检测。设计了两种功能性DNA探针。聚糖探针 (DBCO-GP) 通过代谢寡糖工程 (MOE) 和蛋白质探针 (PP) 靶向蛋白质通过适体识别连接到聚糖上。两种探针之间互补结构域的邻近诱导杂交促进了蛋白质探针的构象变化和 HCR 起始结构域的原位释放。由互补序列(Au-H1 和 Au-H2)修饰的金纳米粒子 (AuNPs) 通过 HCR 自组装成 Au 聚集体,从而将 DNA 信号转换为光声信号。由于光声成像的高对比度和深度穿透,该策略能够原位检测乳腺癌小鼠中的粘蛋白 1 (MUC1) 特异性糖基化,并在衣霉素治疗期间成功监测其动态状态。这种成像技术为研究糖基化对蛋白质结构和功能的影响提供了一个强大的平台,有助于阐明其在疾病过程中的作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号