当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Reduction of C–C π-Bonds via a Cobaltocene-Derived Concerted Proton–Electron Transfer Mediator: Fumarate Hydrogenation as a Model Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/jacs.1c03335 Joseph Derosa 1 , Pablo Garrido-Barros 1 , Jonas C Peters 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/jacs.1c03335 Joseph Derosa 1 , Pablo Garrido-Barros 1 , Jonas C Peters 1
Affiliation
|
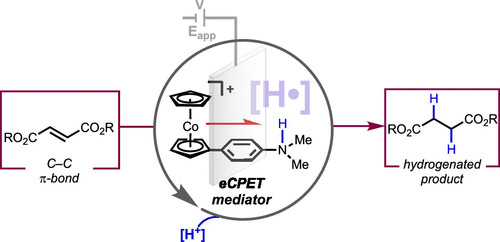
Reductive concerted proton–electron transfer (CPET) is poorly developed for the reduction of C–C π-bonds, including for activated alkenes that can succumb to deleterious pathways (e.g., a competing hydrogen evolution reaction or oligomerization) in a standard electrochemical reduction. We demonstrate herein that selective hydrogenation of the C–C π-bond of fumarate esters can be achieved via electrocatalytic CPET (eCPET) using a CPET mediator comprising cobaltocene with a tethered Brønsted base. High selectivity for electrocatalytic hydrogenation is observed only when the mediator is present. Mechanistic analysis sheds light on two distinct kinetic regimes based on the substrate concentration: low fumarate concentrations operate via rate-limiting CPET followed by an electron-transfer/proton-transfer (ET/PT) step, whereas high concentrations operate via CPET followed by a rate-limiting ET/PT step.
中文翻译:

通过钴茂衍生的协同质子-电子转移介体电催化还原 C-C π-键:富马酸氢化作为模型研究
还原协同质子-电子转移(CPET)在还原 C-C π-键方面的发展很差,包括在标准电化学还原中可能屈服于有害途径(例如,竞争性析氢反应或低聚反应)的活化烯烃。我们在此证明,富马酸酯的 C-C π 键的选择性氢化可以通过电催化 CPET (eCPET) 实现,使用 CPET 介体,该介体包含茂钴和系链布朗斯台德碱。仅当存在介体时才观察到电催化氢化的高选择性。机理分析揭示了基于底物浓度的两种不同的动力学机制:低富马酸盐浓度通过限速 CPET 和电子转移/质子转移 (ET/PT) 步骤进行操作,
更新日期:2021-06-30
中文翻译:

通过钴茂衍生的协同质子-电子转移介体电催化还原 C-C π-键:富马酸氢化作为模型研究
还原协同质子-电子转移(CPET)在还原 C-C π-键方面的发展很差,包括在标准电化学还原中可能屈服于有害途径(例如,竞争性析氢反应或低聚反应)的活化烯烃。我们在此证明,富马酸酯的 C-C π 键的选择性氢化可以通过电催化 CPET (eCPET) 实现,使用 CPET 介体,该介体包含茂钴和系链布朗斯台德碱。仅当存在介体时才观察到电催化氢化的高选择性。机理分析揭示了基于底物浓度的两种不同的动力学机制:低富马酸盐浓度通过限速 CPET 和电子转移/质子转移 (ET/PT) 步骤进行操作,

































 京公网安备 11010802027423号
京公网安备 11010802027423号