当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent-dependent mechanistic aspects for the redox reaction of paraquat in basic solution
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2021-06-18 , DOI: 10.1002/qua.26757 Hua Hou 1 , Baoshan Wang 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2021-06-18 , DOI: 10.1002/qua.26757 Hua Hou 1 , Baoshan Wang 1
Affiliation
|
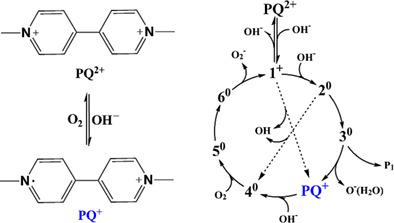
Detailed mechanisms for the redox cycling of paraquat in basic solutions have been revealed computationally. The reduction of paraquat dication (PQ2+) undergoes via the successive additions with two hydroxide (OH−) anions to form the neutralized intermediates, which can decompose to generate the cation radical (PQ+) by releasing either OH or the hydrated O− radical. PQ+ is neutralized by one OH−, converting molecular oxygen into superoxide (O2−) anion to regenerate PQ2+. The reduction of PQ2+ by OH− is an energy-directive process whereas the oxidation of PQ+ prefers an entropy-driving path in which OH− acts as a catalyst. It is found that the Gibbs free-energy reaction paths are strongly solvent dependent. The redox cycle is energetically preferable in the solvents with low dielectric constants. The yellow-blue-transparent color-changing sequence in the clock reaction of paraquat has been understood by means of the electronic absorption spectra of the cations and the neutral intermediates. Atomic radical anion O− is predicted besides the known OH and O2− radicals to stimulate experimental studies on the redox chemistry of paraquat.
中文翻译:

百草枯在碱性溶液中氧化还原反应的溶剂依赖性机理
已通过计算揭示了百草枯在基本溶液中氧化还原循环的详细机制。百草枯二阳离子 ( PQ 2+ )的还原是通过连续添加两个氢氧化物 (OH - ) 阴离子形成中和的中间体,中间体可以通过释放 OH 或水合的 O -分解生成阳离子自由基 ( PQ + )激进。PQ +被一个 OH -中和,将分子氧转化为超氧化物 (O 2 - ) 阴离子以再生PQ 2+。OH -对PQ 2+的还原是一个能量导向过程,而PQ +的氧化更喜欢熵驱动路径,其中 OH -作为催化剂。发现吉布斯自由能反应路径强烈依赖于溶剂。在具有低介电常数的溶剂中,氧化还原循环在能量上是优选的。百草枯的时钟反应中黄-蓝-透明变色顺序已通过阳离子和中性中间体的电子吸收光谱得到理解。除了已知的 OH 和 O 2 -自由基外,还预测原子自由基阴离子 O -以刺激对百草枯氧化还原化学的实验研究。
更新日期:2021-08-20
中文翻译:

百草枯在碱性溶液中氧化还原反应的溶剂依赖性机理
已通过计算揭示了百草枯在基本溶液中氧化还原循环的详细机制。百草枯二阳离子 ( PQ 2+ )的还原是通过连续添加两个氢氧化物 (OH - ) 阴离子形成中和的中间体,中间体可以通过释放 OH 或水合的 O -分解生成阳离子自由基 ( PQ + )激进。PQ +被一个 OH -中和,将分子氧转化为超氧化物 (O 2 - ) 阴离子以再生PQ 2+。OH -对PQ 2+的还原是一个能量导向过程,而PQ +的氧化更喜欢熵驱动路径,其中 OH -作为催化剂。发现吉布斯自由能反应路径强烈依赖于溶剂。在具有低介电常数的溶剂中,氧化还原循环在能量上是优选的。百草枯的时钟反应中黄-蓝-透明变色顺序已通过阳离子和中性中间体的电子吸收光谱得到理解。除了已知的 OH 和 O 2 -自由基外,还预测原子自由基阴离子 O -以刺激对百草枯氧化还原化学的实验研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号