Cancer Cell ( IF 48.8 ) Pub Date : 2021-06-17 , DOI: 10.1016/j.ccell.2021.05.009 Lajos Pusztai 1 , Christina Yau 2 , Denise M Wolf 3 , Hyo S Han 4 , Lili Du 5 , Anne M Wallace 6 , Erica String-Reasor 7 , Judy C Boughey 8 , A Jo Chien 2 , Anthony D Elias 9 , Heather Beckwith 10 , Rita Nanda 11 , Kathy S Albain 12 , Amy S Clark 13 , Kathleen Kemmer 14 , Kevin Kalinsky 15 , Claudine Isaacs 16 , Alexandra Thomas 17 , Rebecca Shatsky 6 , Theresa L Helsten 18 , Andres Forero-Torres 7 , Minetta C Liu 8 , Lamorna Brown-Swigart 3 , Emmanuel F Petricoin 19 , Julia D Wulfkuhle 19 , Smita M Asare 20 , Amy Wilson 20 , Ruby Singhrao 2 , Laura Sit 2 , Gillian L Hirst 2 , Scott Berry 21 , Ashish Sanil 21 , Adam L Asare 20 , Jeffrey B Matthews 2 , Jane Perlmutter 22 , Michelle Melisko 2 , Hope S Rugo 2 , Richard B Schwab 18 , W Fraser Symmans 5 , Doug Yee 10 , Laura J Van't Veer 2 , Nola M Hylton 2 , Angela M DeMichele 13 , Donald A Berry 21 , Laura J Esserman 2
|
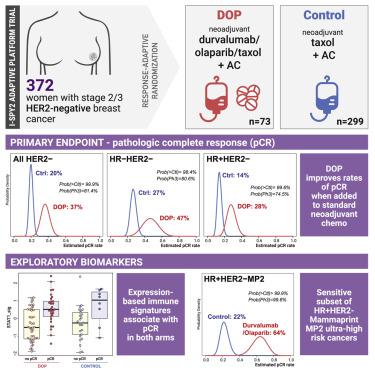
The combination of PD-L1 inhibitor durvalumab and PARP inhibitor olaparib added to standard paclitaxel neoadjuvant chemotherapy (durvalumab/olaparib/paclitaxel [DOP]) was investigated in the phase II I-SPY2 trial of stage II/III HER2-negative breast cancer. Seventy-three participants were randomized to DOP and 299 to standard of care (paclitaxel) control. DOP increased pathologic complete response (pCR) rates in all HER2-negative (20%–37%), hormone receptor (HR)-positive/HER2-negative (14%–28%), and triple-negative breast cancer (TNBC) (27%–47%). In HR-positive/HER2-negative cancers, MammaPrint ultra-high (MP2) cases benefited selectively from DOP (pCR 64% versus 22%), no benefit was seen in MP1 cancers (pCR 9% versus 10%). Overall, 12.3% of patients in the DOP arm experienced immune-related grade 3 adverse events versus 1.3% in control. Gene expression signatures associated with immune response were positively associated with pCR in both arms, while a mast cell signature was associated with non-pCR. DOP has superior efficacy over standard neoadjuvant chemotherapy in HER2-negative breast cancer, particularly in a highly sensitive subset of high-risk HR-positive/HER2-negative patients.
中文翻译:

Durvalumab 联合奥拉帕尼和紫杉醇治疗高危 HER2 阴性 II/III 期乳腺癌:适应性随机 I-SPY2 试验的结果
在 II/III 期 HER2 阴性乳腺癌的 II 期 I-SPY2 试验中,对标准紫杉醇新辅助化疗(durvalumab/olaparib/紫杉醇 [DOP])中添加 PD-L1 抑制剂 durvalumab 和 PARP 抑制剂 olaparib 的组合进行了研究。 73 名参与者被随机分配至 DOP,299 名参与者被随机分配至标准护理(紫杉醇)对照。 DOP 提高了所有 HER2 阴性 (20%–37%)、激素受体 (HR) 阳性/HER2 阴性 (14%–28%) 和三阴性乳腺癌 (TNBC) 的病理完全缓解 (pCR) 率(27%–47%)。在 HR 阳性/HER2 阴性癌症中,MammaPrint 超高 (MP2) 病例选择性地从 DOP 中受益(pCR 64% 与 22%),而在 MP1 癌症中未见获益(pCR 9% 与 10%)。总体而言,DOP 组中有 12.3% 的患者经历了免疫相关的 3 级不良事件,而对照组为 1.3%。与免疫反应相关的基因表达特征与双臂的 pCR 呈正相关,而肥大细胞特征与非 pCR 相关。对于 HER2 阴性乳腺癌,DOP 的疗效优于标准新辅助化疗,特别是对于高危 HR 阳性/HER2 阴性患者的高度敏感亚群。































 京公网安备 11010802027423号
京公网安备 11010802027423号