当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Norstictic Acid Is a Selective Allosteric Transcriptional Regulator
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/jacs.1c03258 Julie M Garlick 1, 2 , Steven M Sturlis 1, 2 , Paul A Bruno 1, 2 , Joel A Yates 3 , Amanda L Peiffer 1, 4 , Yejun Liu 1, 4 , Laura Goo 3 , LiWei Bao 3 , Samantha N De Salle 1, 4 , Giselle Tamayo-Castillo 5 , Charles L Brooks 2, 4, 6 , Sofia D Merajver 3 , Anna K Mapp 1, 2, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-17 , DOI: 10.1021/jacs.1c03258 Julie M Garlick 1, 2 , Steven M Sturlis 1, 2 , Paul A Bruno 1, 2 , Joel A Yates 3 , Amanda L Peiffer 1, 4 , Yejun Liu 1, 4 , Laura Goo 3 , LiWei Bao 3 , Samantha N De Salle 1, 4 , Giselle Tamayo-Castillo 5 , Charles L Brooks 2, 4, 6 , Sofia D Merajver 3 , Anna K Mapp 1, 2, 4
Affiliation
|
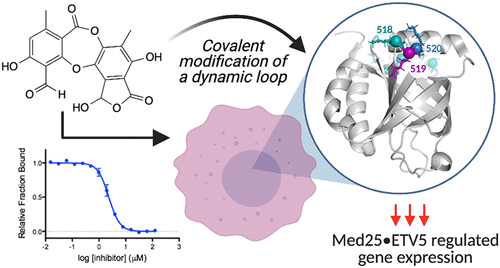
Inhibitors of transcriptional protein–protein interactions (PPIs) have high value both as tools and for therapeutic applications. The PPI network mediated by the transcriptional coactivator Med25, for example, regulates stress-response and motility pathways, and dysregulation of the PPI networks contributes to oncogenesis and metastasis. The canonical transcription factor binding sites within Med25 are large (∼900 Å2) and have little topology, and thus, they do not present an array of attractive small-molecule binding sites for inhibitor discovery. Here we demonstrate that the depsidone natural product norstictic acid functions through an alternative binding site to block Med25–transcriptional activator PPIs in vitro and in cell culture. Norstictic acid targets a binding site comprising a highly dynamic loop flanking one canonical binding surface, and in doing so, it both orthosterically and allosterically alters Med25-driven transcription in a patient-derived model of triple-negative breast cancer. These results highlight the potential of Med25 as a therapeutic target as well as the inhibitor discovery opportunities presented by structurally dynamic loops within otherwise challenging proteins.
中文翻译:

Norstictic Acid 是一种选择性变构转录调节剂
转录蛋白-蛋白相互作用 (PPI) 抑制剂作为工具和治疗应用都具有很高的价值。例如,由转录共激活因子 Med25 介导的 PPI 网络调节应激反应和运动途径,PPI 网络的失调有助于肿瘤发生和转移。 Med25 内的典型转录因子结合位点很大(∼900 Å 2 )并且几乎没有拓扑结构,因此,它们不提供一系列用于抑制剂发现的有吸引力的小分子结合位点。在这里,我们证明了缩西酮天然产物 Norstic Acid 通过替代结合位点发挥作用,在体外和细胞培养中阻断 Med25 转录激活剂 PPI。降雪草酸靶向一个结合位点,该结合位点包含一个典型结合表面侧翼的高度动态环,在此过程中,它在源自患者的三阴性乳腺癌模型中以正构和变构方式改变了 Med25 驱动的转录。这些结果凸显了 Med25 作为治疗靶点的潜力,以及其他具有挑战性的蛋白质中结构动态环所带来的抑制剂发现机会。
更新日期:2021-06-30
中文翻译:

Norstictic Acid 是一种选择性变构转录调节剂
转录蛋白-蛋白相互作用 (PPI) 抑制剂作为工具和治疗应用都具有很高的价值。例如,由转录共激活因子 Med25 介导的 PPI 网络调节应激反应和运动途径,PPI 网络的失调有助于肿瘤发生和转移。 Med25 内的典型转录因子结合位点很大(∼900 Å 2 )并且几乎没有拓扑结构,因此,它们不提供一系列用于抑制剂发现的有吸引力的小分子结合位点。在这里,我们证明了缩西酮天然产物 Norstic Acid 通过替代结合位点发挥作用,在体外和细胞培养中阻断 Med25 转录激活剂 PPI。降雪草酸靶向一个结合位点,该结合位点包含一个典型结合表面侧翼的高度动态环,在此过程中,它在源自患者的三阴性乳腺癌模型中以正构和变构方式改变了 Med25 驱动的转录。这些结果凸显了 Med25 作为治疗靶点的潜力,以及其他具有挑战性的蛋白质中结构动态环所带来的抑制剂发现机会。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号