当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Pesticidal Activities of Pyrimidin-4-amine Derivatives Bearing a 5-(Trifluoromethyl)-1,2,4-oxadiazole Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-06-17 , DOI: 10.1021/acs.jafc.1c00236 Xing-Hai Liu 1 , Yong-Hui Wen 1, 2 , Long Cheng 1, 2 , Tian-Ming Xu 2 , Ning-Jie Wu 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-06-17 , DOI: 10.1021/acs.jafc.1c00236 Xing-Hai Liu 1 , Yong-Hui Wen 1, 2 , Long Cheng 1, 2 , Tian-Ming Xu 2 , Ning-Jie Wu 2
Affiliation
|
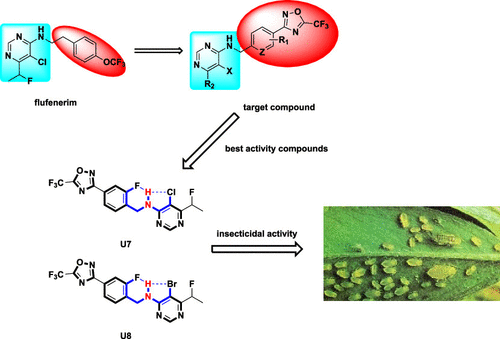
It is important to discover new pesticides with new modes of action because of the increasing evolution of pesticide resistance. In this study, a series of novel pyrimidin-4-amine derivatives containing a 5-(trifluoromethyl)-1,2,4-oxadiazole moiety were designed and synthesized. Their structures were confirmed by 1H NMR, 13C NMR, and HRMS. Bioassays indicated that the 29 compounds synthesized possessed excellent insecticidal activity against Mythimna separata, Aphis medicagini, and Tetranychus cinnabarinus and fungicidal activity against Pseudoperonospora cubensis. Among these pyrimidin-4-amine compounds, 5-chloro-N-(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl)pyrimidin-4-amine (U7) and 5-bromo-N-(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl) pyrimidin-4-amine(U8) had broad-spectrum insecticidal and fungicidal activity. The LC50 values were 3.57 ± 0.42, 4.22 ± 0.47, and 3.14 ± 0.73 mg/L for U7, U8, and flufenerim against M. separata, respectively. The EC50 values were 24.94 ± 2.13, 30.79 ± 2.21, and 3.18 ± 0.21 mg/L for U7, U8, and azoxystrobin against P. cubensis, respectively. The AChE enzymatic activity testing revealed that the enzyme activities of compounds U7, U8, and flufenerim are 0.215, 0.184, and 0.184 U/mg prot, respectively. The molecular docking results of compounds U7, U8, and flufenerim with the AChE model demonstrated the opposite docking mode between compound U7 or U8 and positive control flufenerim in the active site of AChE. The structure–activity relationships are also discussed. This work provided excellent pesticide for further optimization. Density functional theory analysis can potentially be used to design more active compounds.
中文翻译:

带有 5-(三氟甲基)-1,2,4-恶二唑部分的嘧啶-4-胺衍生物的设计、合成和杀虫活性
由于农药耐药性的不断演变,发现具有新作用模式的新农药非常重要。在这项研究中,设计并合成了一系列含有 5-(三氟甲基)-1,2,4-恶二唑部分的新型嘧啶-4-胺衍生物。它们的结构经1 H NMR、13 C NMR 和 HRMS证实。生物测定表明,化合物29对合成具有优异的杀虫活性粘虫,蚜虫medicagini,和朱砂叶螨和杀真菌活性抗霜霉病。在这些嘧啶-4-胺类化合物中,5-氯-N-(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl)pyrimidin-4-amine ( U7 ) and 5-bromo- N -(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl)pyrimidin-4-amine( U8 ) 具有广谱性杀虫和杀真菌活性。U7、U8和氟芬林对分离枝杆菌的 LC 50值分别为 3.57 ± 0.42、4.22 ± 0.47 和 3.14 ± 0.73 mg/L 。对于U7、U8和嘧菌酯,EC 50值分别为 24.94 ± 2.13、30.79 ± 2.21 和 3.18 ± 0.21 mg/L 。, 分别。AChE酶活性测试表明化合物U7、U8和flufenerim的酶活性分别为0.215、0.184和0.184 U/mg prot。化合物U7、U8和氟芬林与AChE模型的分子对接结果表明,化合物U7或U8与AChE活性位点的阳性对照氟芬林之间的对接模式相反。还讨论了构效关系。这项工作为进一步优化提供了极好的农药。密度泛函理论分析可潜在地用于设计更具活性的化合物。
更新日期:2021-06-30
中文翻译:

带有 5-(三氟甲基)-1,2,4-恶二唑部分的嘧啶-4-胺衍生物的设计、合成和杀虫活性
由于农药耐药性的不断演变,发现具有新作用模式的新农药非常重要。在这项研究中,设计并合成了一系列含有 5-(三氟甲基)-1,2,4-恶二唑部分的新型嘧啶-4-胺衍生物。它们的结构经1 H NMR、13 C NMR 和 HRMS证实。生物测定表明,化合物29对合成具有优异的杀虫活性粘虫,蚜虫medicagini,和朱砂叶螨和杀真菌活性抗霜霉病。在这些嘧啶-4-胺类化合物中,5-氯-N-(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl)pyrimidin-4-amine ( U7 ) and 5-bromo- N -(2-fluoro-4-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)benzyl)-6-(1-fluoroethyl)pyrimidin-4-amine( U8 ) 具有广谱性杀虫和杀真菌活性。U7、U8和氟芬林对分离枝杆菌的 LC 50值分别为 3.57 ± 0.42、4.22 ± 0.47 和 3.14 ± 0.73 mg/L 。对于U7、U8和嘧菌酯,EC 50值分别为 24.94 ± 2.13、30.79 ± 2.21 和 3.18 ± 0.21 mg/L 。, 分别。AChE酶活性测试表明化合物U7、U8和flufenerim的酶活性分别为0.215、0.184和0.184 U/mg prot。化合物U7、U8和氟芬林与AChE模型的分子对接结果表明,化合物U7或U8与AChE活性位点的阳性对照氟芬林之间的对接模式相反。还讨论了构效关系。这项工作为进一步优化提供了极好的农药。密度泛函理论分析可潜在地用于设计更具活性的化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号