Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2021-06-17 , DOI: 10.1016/j.apcata.2021.118269 Cen Tang , Fangru Zhou , Yang Wen , Yumeng Xu , Aiping Jia , Mengfei Luo , Jiqing Lu
|
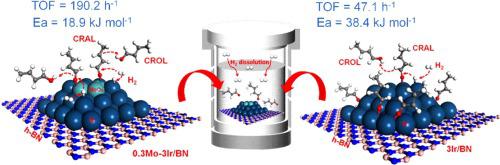
A series of MoOx-promoted Ir/BN catalysts were tested for liquid phase selective hydrogenation of crotonaldehyde. The MoOx-promotion could significantly improve the reactivity up to 5-fold. Such improvement was mainly due to the formation of Ir-MoOx interfacial sites in the promoted catalyst, which accelerated the adsorption and activation of crotonaldehyde molecule as evidenced by a much lower activation energy (18.9 kJ mol−1 on the 0.3Mo-3Ir/BN versus 38.4 kJ mol−1 on the 3Ir/BN). The kinetic results revealed negative reaction order of crotonaldehyde (up to -1.1) and high reaction order of H2 (up to 2.2), indicating the high coverage of crotonaldehyde on the catalyst surface suppressed the adsorption of H2 due to its low solubility in the liquid phase. Therefore, the adsorption and activation of H2 on the catalyst might be the rate determining step. However, the preferred adsorption of crotonaldehyde on the Ir-MoOx interface alleviated the suppression of H2 adsorption, which was another reason for the higher activity.
中文翻译:

金属-促进剂界面对巴豆醛在 Ir-MoO x /BN 催化剂上液相选择性加氢的作用
测试了一系列 MoO x促进的 Ir/BN 催化剂用于巴豆醛的液相选择性加氢。MoO x促进可以显着提高反应性高达 5 倍。这种改进主要是由于在促进催化剂中形成了 Ir-MoO x界面位点,这加速了巴豆醛分子的吸附和活化,这可以通过低得多的活化能(18.9 kJ mol -1在 0.3Mo-3Ir/ BN 与3Ir/BN 上的38.4 kJ mol -1)。动力学结果显示巴豆醛的负反应级数(高达 -1.1)和 H 2 的高反应级数(高达 2.2),表明巴豆醛在催化剂表面的高覆盖率抑制了 H 2的吸附,因为它在液相中的溶解度低。因此,催化剂上H 2的吸附和活化可能是速率决定步骤。然而,巴豆醛在 Ir-MoO x界面上的优先吸附减轻了对 H 2吸附的抑制,这是较高活性的另一个原因。































 京公网安备 11010802027423号
京公网安备 11010802027423号