European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-06-17 , DOI: 10.1016/j.ejmech.2021.113629 Cheng-Jun Wu 1 , Jia-Qiang Wu 1 , Yunfei Hu 1 , Suyun Pu 1 , Yuying Lin 1 , Zimai Zeng 1 , Jinhui Hu 1 , Wen-Hua Chen 1
|
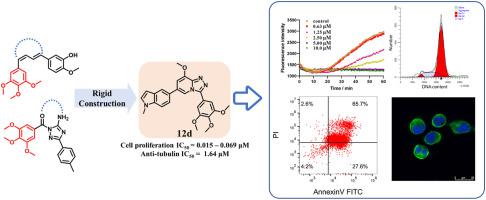
A series of indole-based [1,2,4]triazolo [4,3-a]pyridine derivatives was designed and synthesized as novel microtubulin polymerization inhibitors by using a conformational restriction strategy. These compounds exhibited moderate to potent anti-proliferative activities against a panel of cancer cell lines (HeLa, A549, MCF-7 and HCT116). Among them, compound 12d featuring a N-methyl-5-indolyl substituent at the C-6 position of the [1,2,4]triazolo [4,3-a]pyridine core exhibited the highest antiproliferative activity with the IC50 values ranging from 15 to 69 nM, and remarkable inhibitory effect on tubulin polymerization with an IC50 value of 1.64 μM. Mechanistic studies revealed that compound 12d induced cellular apoptosis and cell cycle arrest at the G2/M phase in a dose-dependent fashion. Moreover, compound 12d significantly suppressed wound closure and disturbed microtubule networks.
中文翻译:

作为新型微管聚合抑制剂的吲哚基[1,2,4]三唑并[4,3-a]吡啶衍生物的设计、合成和生物学评价
通过使用构象限制策略,设计并合成了一系列基于吲哚的 [1,2,4] 三唑并 [4,3- a ] 吡啶衍生物作为新型微管蛋白聚合抑制剂。这些化合物对一组癌细胞系(HeLa、A549、MCF-7 和 HCT116)表现出中等至强效的抗增殖活性。其中,在[1,2,4]三唑并[4,3- a ]吡啶核心的C- 6位具有N-甲基-5-吲哚基取代基的化合物12d表现出最高的抗增殖活性,IC 50值范围从 15 到 69 nM,对微管蛋白聚合有显着的抑制作用,IC 50值为 1.64 μM。机理研究表明,化合物12d以剂量依赖性方式在G 2 /M 期诱导细胞凋亡和细胞周期停滞。此外,化合物12d显着抑制伤口闭合并扰乱微管网络。






























 京公网安备 11010802027423号
京公网安备 11010802027423号