当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-induced iodine-anion-catalyzed decarboxylative/deaminative C–H alkylation of enamides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-6-7 , DOI: 10.1039/d1qo00660f Jia-Xin Wang 1, 2, 3, 4, 5 , Ya-Ting Wang 1, 2, 3, 4, 5 , Hao Zhang 1, 2, 3, 4, 5 , Ming-Chen Fu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-6-7 , DOI: 10.1039/d1qo00660f Jia-Xin Wang 1, 2, 3, 4, 5 , Ya-Ting Wang 1, 2, 3, 4, 5 , Hao Zhang 1, 2, 3, 4, 5 , Ming-Chen Fu 1, 2, 3, 4, 5
Affiliation
|
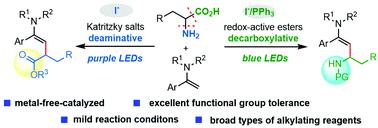
A visible-light-induced iodine-anion-catalyzed C–H stereoselective alkylation of enamides has been developed. Redox-active esters and Katritzky salts of amino acids were found to be amenable for decarboxylative/deaminative cross-coupling reactions, delivering various functionalized enamides with excellent functional group tolerance. The reaction proceeded via photoactivation of the transiently assembled chromophores, avoiding the use of exogenous precious photoredox catalysts.
中文翻译:

可见光诱导的碘阴离子催化的烯酰胺的脱羧/脱氨基C-H烷基化
已开发出可见光诱导的碘阴离子催化的烯酰胺的 C-H 立体选择性烷基化。发现氧化还原活性酯和氨基酸的卡特里茨基盐适用于脱羧/脱氨基交叉偶联反应,提供具有优异官能团耐受性的各种官能化烯酰胺。该反应通过瞬时组装的发色团的光活化进行,避免使用外源性珍贵的光氧化还原催化剂。
更新日期:2021-06-17
中文翻译:

可见光诱导的碘阴离子催化的烯酰胺的脱羧/脱氨基C-H烷基化
已开发出可见光诱导的碘阴离子催化的烯酰胺的 C-H 立体选择性烷基化。发现氧化还原活性酯和氨基酸的卡特里茨基盐适用于脱羧/脱氨基交叉偶联反应,提供具有优异官能团耐受性的各种官能化烯酰胺。该反应通过瞬时组装的发色团的光活化进行,避免使用外源性珍贵的光氧化还原催化剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号