当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of Selective Production of Hydrogen Peroxide by Electrochemical Oxygen Reduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-16 , DOI: 10.1021/jacs.1c02186 Xunhua Zhao 1 , Yuanyue Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-16 , DOI: 10.1021/jacs.1c02186 Xunhua Zhao 1 , Yuanyue Liu 1
Affiliation
|
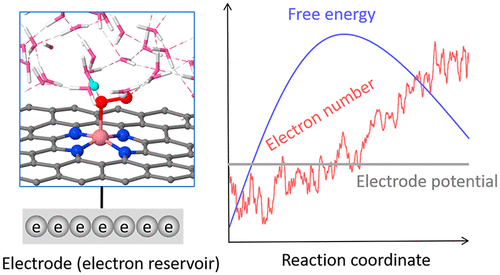
Oxygen reduction reaction (ORR) is one of the most important electrochemical reactions. Starting from a common reaction intermediate *–O–OH, the ORR splits into two pathways, either producing hydrogen peroxide (H2O2) by breaking the *–O bond or leading to water formation by breaking the O–OH bond. However, it is puzzling why many catalysts, despite the strong thermodynamic preference for the O–OH breaking, exhibit high selectivity for hydrogen peroxide. Moreover, the selectivity is dependent on the potential and pH, which remain not understood. Here we develop an advanced first-principles model for effective calculation of the electrochemical reaction kinetics at the solid–water interface, which were not accessible by conventional models. Using this model to study representative catalysts for H2O2 production, we find that breaking the O–OH bond can have a higher energy barrier than breaking *–O, due to the rigidity of the O–OH bond. Importantly, we reveal that the selectivity dependence on potential and pH is rooted into the proton affinity to the former/later O in *–O–OH. For single cobalt atom catalyst, decreasing potential promotes proton adsorption to the former O, thereby increasing the H2O2 selectivity. In contrast, for the carbon catalyst, the proton prefers the latter O, resulting in a lower H2O2 selectivity in acid condition. These findings explain the experiments and highlight the kinetic origins of the selectivity. Our work improves the understanding of ORR by uncovering the proton affinity as a new factor and provides a new model to effectively simulate the atomic-level kinetics of heterogeneous electrochemistry.
中文翻译:

电化学氧还原选择性生产过氧化氢的起源
氧还原反应(ORR)是最重要的电化学反应之一。从常见的反应中间体 *–O–OH 开始,ORR 分成两条途径,要么通过破坏 *–O 键产生过氧化氢 (H 2 O 2 ),要么通过破坏 O–OH 键形成水。然而,令人费解的是,为什么许多催化剂尽管对 O-OH 断裂具有强烈的热力学偏好,却对过氧化氢表现出高选择性。此外,选择性取决于电势和 pH 值,目前尚不清楚。在这里,我们开发了一种先进的第一原理模型,用于有效计算固水界面的电化学反应动力学,这是传统模型无法实现的。使用该模型研究用于生产H 2 O 2的代表性催化剂,我们发现由于O-OH键的刚性,破坏O-OH键比破坏*-O具有更高的能垒。重要的是,我们揭示了选择性对电位和 pH 值的依赖性根源于 *–O–OH 中前/后 O 的质子亲和力。对于单钴原子催化剂,降低电势会促进质子对前O的吸附,从而提高H 2 O 2的选择性。相反,对于碳催化剂,质子更喜欢后者的O,导致酸性条件下H 2 O 2选择性较低。这些发现解释了实验并强调了选择性的动力学起源。我们的工作通过发现质子亲和力作为一个新因素提高了对 ORR 的理解,并提供了一个新模型来有效模拟异质电化学的原子级动力学。
更新日期:2021-06-30
中文翻译:

电化学氧还原选择性生产过氧化氢的起源
氧还原反应(ORR)是最重要的电化学反应之一。从常见的反应中间体 *–O–OH 开始,ORR 分成两条途径,要么通过破坏 *–O 键产生过氧化氢 (H 2 O 2 ),要么通过破坏 O–OH 键形成水。然而,令人费解的是,为什么许多催化剂尽管对 O-OH 断裂具有强烈的热力学偏好,却对过氧化氢表现出高选择性。此外,选择性取决于电势和 pH 值,目前尚不清楚。在这里,我们开发了一种先进的第一原理模型,用于有效计算固水界面的电化学反应动力学,这是传统模型无法实现的。使用该模型研究用于生产H 2 O 2的代表性催化剂,我们发现由于O-OH键的刚性,破坏O-OH键比破坏*-O具有更高的能垒。重要的是,我们揭示了选择性对电位和 pH 值的依赖性根源于 *–O–OH 中前/后 O 的质子亲和力。对于单钴原子催化剂,降低电势会促进质子对前O的吸附,从而提高H 2 O 2的选择性。相反,对于碳催化剂,质子更喜欢后者的O,导致酸性条件下H 2 O 2选择性较低。这些发现解释了实验并强调了选择性的动力学起源。我们的工作通过发现质子亲和力作为一个新因素提高了对 ORR 的理解,并提供了一个新模型来有效模拟异质电化学的原子级动力学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号