当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comprehensive Study about the Photolysis of Nitrates on Mineral Oxides
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.est.1c02182 Qingxin Ma 1, 2, 3 , Cheng Zhong 1, 3 , Jinzhu Ma 1, 2, 3 , Chunxiang Ye 4 , Yaqi Zhao 1, 3 , Yuan Liu 1, 3 , Peng Zhang 1, 3 , Tianzeng Chen 1 , Chang Liu 5 , Biwu Chu 1, 2, 3 , Hong He 1, 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.est.1c02182 Qingxin Ma 1, 2, 3 , Cheng Zhong 1, 3 , Jinzhu Ma 1, 2, 3 , Chunxiang Ye 4 , Yaqi Zhao 1, 3 , Yuan Liu 1, 3 , Peng Zhang 1, 3 , Tianzeng Chen 1 , Chang Liu 5 , Biwu Chu 1, 2, 3 , Hong He 1, 2, 3
Affiliation
|
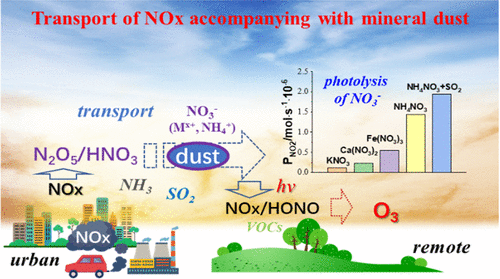
Nitrates formed on mineral dust through heterogeneous reactions in high NOx areas can undergo photolysis to regenerate NOx and potentially interfere in the photochemistry in the downwind low NOx areas. However, little is known about such renoxification processes. In this study, photolysis of various nitrates on different mineral oxides was comprehensively investigated in a flow reactor and in situ diffuse reflectance Fourier-transform infrared spectroscopy (in situ DRIFTS). TiO2 was found much more reactive than Al2O3 and SiO2 with both NO2 and HONO as the two major photolysis products. The yields of NO2 and HONO depend on the cation basicity of the nitrate salts or the acidity of particles. As such, NH4NO3 is much more productive than other nitrates like Fe(NO3)3, Ca(NO3)2, and KNO3. SO2 and water vapor promote the photodegradation by increasing the surface acidity due to the photoinduced formation of H2SO4/sulfate and H+, respectively. O2 enables the photo-oxidation of NOx to regenerate nitrate and thus inhibits the NOx yield. Overall, our results demonstrated that the photolysis of nitrate can be accelerated under complex air pollution conditions, which are helpful for understanding the transformation of nitrate and the nitrogen cycle in the atmosphere.
中文翻译:

硝酸盐在矿物氧化物上光解的综合研究
通过高NO x区域的异质反应在矿物粉尘上形成的硝酸盐可以进行光解以再生NO x并可能干扰顺风低NO x区域的光化学。然而,人们对这种再氧化过程知之甚少。在这项研究中,在流动反应器和原位漫反射傅里叶变换红外光谱(原位 DRIFTS)中全面研究了各种硝酸盐在不同矿物氧化物上的光解。发现TiO 2比 Al 2 O 3和 SiO 2具有更高的反应性,NO 2和 HONO 作为两种主要的光解产物。NO 2的产率和 HONO 取决于硝酸盐的阳离子碱度或颗粒的酸度。因此,NH 4 NO 3比其他硝酸盐如 Fe(NO 3 ) 3、Ca(NO 3 ) 2和 KNO 3 的生产率高得多。SO 2和水蒸气分别通过光诱导形成 H 2 SO 4 /硫酸盐和 H +增加表面酸度来促进光降解。O 2使 NO x光氧化再生硝酸盐,从而抑制 NO x屈服。总体而言,我们的研究结果表明,在复杂的空气污染条件下可以加速硝酸盐的光解,这有助于理解大气中硝酸盐的转化和氮循环。
更新日期:2021-07-06
中文翻译:

硝酸盐在矿物氧化物上光解的综合研究
通过高NO x区域的异质反应在矿物粉尘上形成的硝酸盐可以进行光解以再生NO x并可能干扰顺风低NO x区域的光化学。然而,人们对这种再氧化过程知之甚少。在这项研究中,在流动反应器和原位漫反射傅里叶变换红外光谱(原位 DRIFTS)中全面研究了各种硝酸盐在不同矿物氧化物上的光解。发现TiO 2比 Al 2 O 3和 SiO 2具有更高的反应性,NO 2和 HONO 作为两种主要的光解产物。NO 2的产率和 HONO 取决于硝酸盐的阳离子碱度或颗粒的酸度。因此,NH 4 NO 3比其他硝酸盐如 Fe(NO 3 ) 3、Ca(NO 3 ) 2和 KNO 3 的生产率高得多。SO 2和水蒸气分别通过光诱导形成 H 2 SO 4 /硫酸盐和 H +增加表面酸度来促进光降解。O 2使 NO x光氧化再生硝酸盐,从而抑制 NO x屈服。总体而言,我们的研究结果表明,在复杂的空气污染条件下可以加速硝酸盐的光解,这有助于理解大气中硝酸盐的转化和氮循环。































 京公网安备 11010802027423号
京公网安备 11010802027423号