当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Water Splitting Reaction Catalyzed by Ion-Exchanged Salts of Potassium Poly(heptazine imide) 2D Materials
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.jpcc.1c03947 Sudhir K Sahoo 1 , Ivo F Teixeira 2, 3 , Aakash Naik 1 , Julian Heske 1, 2 , Daniel Cruz 4, 5 , Markus Antonietti 2 , Aleksandr Savateev 2 , Thomas D Kühne 1, 6
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.jpcc.1c03947 Sudhir K Sahoo 1 , Ivo F Teixeira 2, 3 , Aakash Naik 1 , Julian Heske 1, 2 , Daniel Cruz 4, 5 , Markus Antonietti 2 , Aleksandr Savateev 2 , Thomas D Kühne 1, 6
Affiliation
|
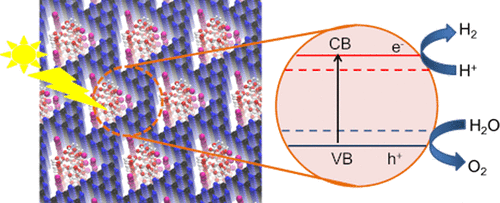
Potassium poly (heptazine imide) (K-PHI), a crystalline two-dimensional carbon–nitride material, is an active photocatalyst for water splitting. The potassium ions in K-PHI can be exchanged with other ions to change the properties of the material and eventually to design the catalysts. We report here the electronic structures of several ion-exchanged salts of K-PHI (K, H, Au, Ru, and Mg) and their feasibility as water splitting photocatalysts, which were determined by density functional theory (DFT) calculations. The DFT results are complemented by experiments where the performances in the photocatalytic hydrogen evolution reaction (HER) were recorded. We show that due to its narrow band gap, Ru-PHI is not a suitable photocatalyst. The water oxidation potentials are straddled between the band edge potentials of H-PHI, Au-PHI, and Mg-PHI; thus, these are active photocatalysts for both the oxygen and hydrogen evolution reactions, whereas K-PHI is active only for the HER. The experimental data show that these are active HER photocatalysts, in agreement with the DFT results. Furthermore, Mg-PHI has shown remarkable performance in the HER, with a rate of 539 μmol/(h·g) and a quantum efficiency of 7.14% at 410 nm light irradiation, which could be due to activation of the water molecule upon adsorption, as predicted by our DFT calculations.
中文翻译:

聚(庚嗪酰亚胺)钾离子交换盐2D材料催化光催化分解水反应
聚(庚嗪酰亚胺)钾(K-PHI)是一种结晶二维碳氮化物材料,是一种用于水分解的活性光催化剂。K-PHI 中的钾离子可以与其他离子交换以改变材料的性质并最终设计催化剂。我们在此报告了 K-PHI(K、H、Au、Ru 和 Mg)的几种离子交换盐的电子结构及其作为水分解光催化剂的可行性,这是通过密度泛函理论 (DFT) 计算确定的。DFT 结果由记录光催化析氢反应 (HER) 性能的实验补充。我们表明,由于其窄带隙,Ru-PHI 不是合适的光催化剂。水氧化电位横跨在 H-PHI、Au-PHI 和 Mg-PHI 的带边电位之间;因此,这些是用于析氧和析氢反应的活性光催化剂,而 K-PHI 仅对 HER 有活性。实验数据表明,这些是活性 HER 光催化剂,与 DFT 结果一致。此外,Mg-PHI 在 HER 中表现出卓越的性能,在 410 nm 光照射下的速率为 539 μmol/(h·g),量子效率为 7.14%,这可能是由于吸附时水分子被活化,正如我们的 DFT 计算所预测的那样。
更新日期:2021-07-01
中文翻译:

聚(庚嗪酰亚胺)钾离子交换盐2D材料催化光催化分解水反应
聚(庚嗪酰亚胺)钾(K-PHI)是一种结晶二维碳氮化物材料,是一种用于水分解的活性光催化剂。K-PHI 中的钾离子可以与其他离子交换以改变材料的性质并最终设计催化剂。我们在此报告了 K-PHI(K、H、Au、Ru 和 Mg)的几种离子交换盐的电子结构及其作为水分解光催化剂的可行性,这是通过密度泛函理论 (DFT) 计算确定的。DFT 结果由记录光催化析氢反应 (HER) 性能的实验补充。我们表明,由于其窄带隙,Ru-PHI 不是合适的光催化剂。水氧化电位横跨在 H-PHI、Au-PHI 和 Mg-PHI 的带边电位之间;因此,这些是用于析氧和析氢反应的活性光催化剂,而 K-PHI 仅对 HER 有活性。实验数据表明,这些是活性 HER 光催化剂,与 DFT 结果一致。此外,Mg-PHI 在 HER 中表现出卓越的性能,在 410 nm 光照射下的速率为 539 μmol/(h·g),量子效率为 7.14%,这可能是由于吸附时水分子被活化,正如我们的 DFT 计算所预测的那样。

































 京公网安备 11010802027423号
京公网安备 11010802027423号