Chemico-Biological Interactions ( IF 4.7 ) Pub Date : 2021-06-15 , DOI: 10.1016/j.cbi.2021.109540
Kameliya Anichina 1 , Maria Argirova 2 , Rumyana Tzoneva 3 , Veselina Uzunova 3 , Anelia Mavrova 1 , Dimitar Vuchev 4 , Galya Popova-Daskalova 4 , Filip Fratev 5 , Maya Guncheva 2 , Denitsa Yancheva 2
|
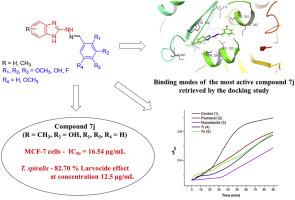
In the present study, fifteen benzimidazolyl-2-hydrazones 7a-7o of fluoro-, hydroxy- and methoxy-substituted benzaldehydes and 1,3-benzodioxole-5-carbaldehyde were synthesized and their structure was identified by IR, NMR, and elemental analysis. The compounds 7j 2-(3-hydroxybenzylidene)-1-(5(6)-methyl-1H-benzimidazol-2-yl)hydrazone and 7i 2-(3-hydroxybenzylidene)-1-(1H-benzimidazol-2-yl)hydrazone have exerted the strongest anthelmintic activity (100% after 24 h incubation period at 37 °C) against isolated muscle larvae of Trichinella spiralis in an in vitro experiment. The in vitro cytotoxicity assay towards MCF-7 breast cancer cells and mouse embryo fibroblasts 3T3 showed that the studied benzimidazolyl-2-hydrazones exhibit low to moderate cytotoxic effects. The ability of the studied benzimidazolyl-2-hydrazones to modulate microtubule polymerization was confirmed and suggested that their anthelmintic action is mediated through inhibition of the tubulin polymerization likewise the other known benzimidazole anthelmitics. It was also shown that the four most promising benzimidazolyl-2-hydrazones do not affect significantly the AChE activity even at high tested concentration, thus indicating that they do not have the potential for neurotoxic effects. The binding mode of compounds 7j and 7n in the colchicine-binding site of tubulin were clarified by molecular docking simulations. Taken together, these results demonstrate that for the synthesized benzimidazole derivatives the anthelmintic activity against T. spiralis and the inhibition of tubulin polymerization are closely related.
中文翻译:

1H-苯并咪唑-2-基腙作为微管蛋白靶向剂:合成、结构表征、抗蠕虫活性和抗 MCF-7 乳腺癌细胞增殖活性和分子对接研究
在本研究中,合成了 15 个苯并咪唑基-2-腙7a-7o的氟-、羟基-和甲氧基-取代的苯甲醛和 1,3-苯并二氧戊环-5-甲醛,并通过红外、核磁共振和元素分析鉴定了它们的结构. 化合物7j 2-(3-羟基苯亚甲基)-1-(5(6)-甲基-1 H-苯并咪唑-2-基)腙和7i 2-(3-羟基苯亚甲基)-1-(1 H-苯并咪唑-2基)腙都产生最强的驱虫活性(100%后,在37℃)对的分离的肌幼虫24小时温育期旋毛虫在体外实验。在体外向细胞毒性测定MCF-7乳腺癌细胞和小鼠胚胎成纤维细胞3T3表明所研究的苯并咪唑基-2-腙表现出低至中度的细胞毒性作用。所研究的苯并咪唑基-2-腙调节微管聚合的能力得到证实,并表明它们的驱虫作用是通过抑制微管蛋白聚合来介导的,与其他已知的苯并咪唑驱虫药一样。还表明,四种最有希望的苯并咪唑基-2-腙即使在高测试浓度下也不会显着影响 AChE 活性,因此表明它们不具有神经毒性作用的潜力。化合物7j和7n的结合方式通过分子对接模拟阐明了微管蛋白秋水仙碱结合位点中的 综上所述,这些结果表明合成的苯并咪唑衍生物对螺旋线虫的驱虫活性和微管蛋白聚合的抑制密切相关。

































 京公网安备 11010802027423号
京公网安备 11010802027423号