European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-06-15 , DOI: 10.1016/j.ejmech.2021.113644 Tengfei Xu 1 , Yaping Xue 1 , Jielian Lu 1 , Chuanfei Jin 1
|
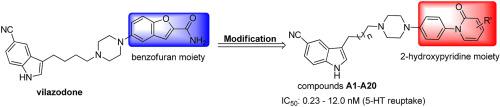
A series of novel 1-(4-(piperazin-1-yl)phenyl)pyridin-2(1H)-one derivatives were synthesized and evaluated for their serotonin (5-HT) reuptake inhibitory activity. The results in vitro indicated that most of the evaluated compounds displayed potent 5-HT reuptake inhibition. The most promising compound A20 was stable in human liver microsomes and possessed good pharmacokinetic properties. Antidepressant study in vivo of the compound A20 showed that A20 could potently antagonize the p-chloroamphetamine (PCA)-induced depletion of serotonin in hypothalamus and reduce immobility times in the rat forced swimming test (FST).
中文翻译:

作为潜在 SSRIs 的 1-(4-(piperazin-1-yl)phenyl)pyridin-2(1H)-one 衍生物的合成和生物学评价
合成了一系列新型 1-(4-(piperazin-1-yl)phenyl)pyridin-2(1H)-one 衍生物,并对其血清素 (5-HT) 再摄取抑制活性进行了评估。体外结果表明,大多数评估的化合物显示出有效的 5-HT 再摄取抑制。最有前途的化合物A20在人肝微粒体中稳定,具有良好的药代动力学特性。化合物A20 的体内抗抑郁研究表明,A20可以有效地拮抗对氯苯丙胺 (PCA) 诱导的下丘脑血清素消耗,并减少大鼠强迫游泳试验 (FST) 中的不动时间。






























 京公网安备 11010802027423号
京公网安备 11010802027423号