当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics Perspective on the Stepwise Conversion of Methane to Methanol over Cu-Exchanged SSZ-13
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-11 , DOI: 10.1021/acscatal.1c00691 Florian Göltl 1 , Saurabh Bhandari 1 , Manos Mavrikakis 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-11 , DOI: 10.1021/acscatal.1c00691 Florian Göltl 1 , Saurabh Bhandari 1 , Manos Mavrikakis 1
Affiliation
|
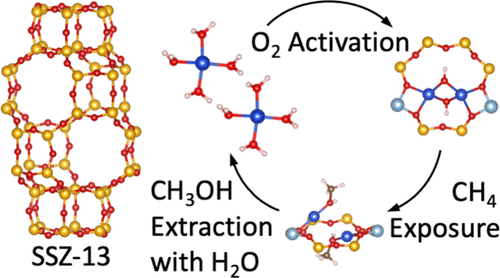
Transition-metal exchanged zeolites are known to convert methane to methanol with high selectivity, in a stepwise process, involving exposure to oxidants, followed by exposure to methane, and finally by exposure to water vapor. However, a comprehensive theoretical study on the nature of the possible active sites and their respective changes during this stepwise process is still lacking. Here, we use a combination of density functional theory calculations in its generalized-gradient approximation (DFT-GGA) and post-DFT methods to identify the thermodynamically preferred sites in Cu-exchanged zeolite SSZ-13 during the stepwise conversion of methane to methanol. We develop a thermodynamic model for an extensive set of possible active sites, that is, Cu monomers, dimers, and trimers, which are anchored in different ring structures and supported by a series of different local Al distributions. Subsequently, phase diagrams are constructed and used to identify thermodynamically favored sites at each step during the stepwise conversion of methane to methanol. We find that during exposure to O2, hydroxylated dimers—Cu2O2H2 and, depending on the local Al configuration, Cu2OH—are preferred. Upon exposure to methane, site-bound methanol molecules are formed. With the subsequent increase in water vapor pressure, a thermodynamic preference for monoatomic Cu and the release of methanol are observed. Furthermore, we compare our predicted results to experimental measurements published in the literature and find close agreement in terms of Cu coordination number and bond distances for some of the sites considered. We expect that the insights obtained here can be used to improve our understanding of the reaction mechanism and to optimize the stepwise conversion of methane to methanol.
中文翻译:

通过 Cu 交换的 SSZ-13 将甲烷逐步转化为甲醇的热力学观点
已知过渡金属交换沸石以高选择性将甲烷转化为甲醇,分步过程包括暴露于氧化剂,然后暴露于甲烷,最后暴露于水蒸气。然而,关于可能的活性位点的性质及其在这个逐步过程中各自的变化,仍然缺乏全面的理论研究。在这里,我们在其广义梯度近似 (DFT-GGA) 和后 DFT 方法中使用密度泛函理论计算的组合来确定在甲烷到甲醇的逐步转化过程中 Cu 交换沸石 SSZ-13 中的热力学优选位点。我们为大量可能的活性位点开发了一个热力学模型,即 Cu 单体、二聚体和三聚体,它们锚定在不同的环结构中,并由一系列不同的局部铝分布支撑。随后,构建相图并用于确定在甲烷逐步转化为甲醇过程中每一步的热力学有利位点。我们发现在暴露于 O如图2所示,优选羟基化二聚体-Cu 2 O 2 H 2和取决于局部Al构型的Cu 2 OH-。接触甲烷后,会形成位点结合的甲醇分子。随着水蒸气压的随后增加,观察到单原子铜的热力学偏好和甲醇的释放。此外,我们将我们的预测结果与文献中发表的实验测量结果进行了比较,并发现所考虑的一些位点在 Cu 配位数和键距方面非常一致。我们希望这里获得的见解可用于提高我们对反应机理的理解,并优化甲烷向甲醇的逐步转化。
更新日期:2021-07-02
中文翻译:

通过 Cu 交换的 SSZ-13 将甲烷逐步转化为甲醇的热力学观点
已知过渡金属交换沸石以高选择性将甲烷转化为甲醇,分步过程包括暴露于氧化剂,然后暴露于甲烷,最后暴露于水蒸气。然而,关于可能的活性位点的性质及其在这个逐步过程中各自的变化,仍然缺乏全面的理论研究。在这里,我们在其广义梯度近似 (DFT-GGA) 和后 DFT 方法中使用密度泛函理论计算的组合来确定在甲烷到甲醇的逐步转化过程中 Cu 交换沸石 SSZ-13 中的热力学优选位点。我们为大量可能的活性位点开发了一个热力学模型,即 Cu 单体、二聚体和三聚体,它们锚定在不同的环结构中,并由一系列不同的局部铝分布支撑。随后,构建相图并用于确定在甲烷逐步转化为甲醇过程中每一步的热力学有利位点。我们发现在暴露于 O如图2所示,优选羟基化二聚体-Cu 2 O 2 H 2和取决于局部Al构型的Cu 2 OH-。接触甲烷后,会形成位点结合的甲醇分子。随着水蒸气压的随后增加,观察到单原子铜的热力学偏好和甲醇的释放。此外,我们将我们的预测结果与文献中发表的实验测量结果进行了比较,并发现所考虑的一些位点在 Cu 配位数和键距方面非常一致。我们希望这里获得的见解可用于提高我们对反应机理的理解,并优化甲烷向甲醇的逐步转化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号