当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the scope of the Babler–Dauben oxidation: 1,3-oxidative transposition of secondary allylic alcohols
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-08-06 11:04:24 Patrick M. Killoran, Steven B. Rossington, James A. Wilkinson, John A. Hadfield
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-08-06 11:04:24 Patrick M. Killoran, Steven B. Rossington, James A. Wilkinson, John A. Hadfield
|
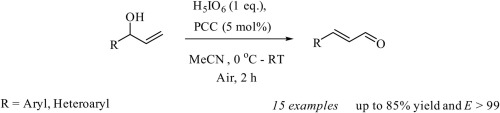
We report the catalytic chromium-mediated oxidation of secondary allylic alcohols to give α,β-unsaturated aldehydes with exclusive (E)-stereoselectivity. This facile procedure employs catalytic PCC (5mol%) and periodic acid (H5IO6) as a co-oxidant. This transformation occurs specifically with aromatic substituted allyl alcohols containing both electron withdrawing and electron donating substituents as well as a range of functional groups.
中文翻译:

扩大Babler–Dauben氧化范围:仲烯丙基醇的1,3-氧化转座
我们报告了催化铬介导的仲烯丙基醇的氧化,以产生具有排他性(E)-立体选择性的α,β-不饱和醛。该简便的方法使用催化PCC(5mol%)和高碘酸(H 5 IO 6)作为助氧化剂。这种转变特别发生在含有吸电子和供电子取代基以及一系列官能团的芳族取代的烯丙醇上。
更新日期:2016-08-07
中文翻译:

扩大Babler–Dauben氧化范围:仲烯丙基醇的1,3-氧化转座
我们报告了催化铬介导的仲烯丙基醇的氧化,以产生具有排他性(E)-立体选择性的α,β-不饱和醛。该简便的方法使用催化PCC(5mol%)和高碘酸(H 5 IO 6)作为助氧化剂。这种转变特别发生在含有吸电子和供电子取代基以及一系列官能团的芳族取代的烯丙醇上。































 京公网安备 11010802027423号
京公网安备 11010802027423号