当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Nucleoside Antibiotics Amicetin, Plicacetin, and Cytosaminomycin A—D
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-06-07 , DOI: 10.1002/cjoc.202100284 Jiqiang Fu 1 , Peng Xu 1, 2 , Biao Yu 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-06-07 , DOI: 10.1002/cjoc.202100284 Jiqiang Fu 1 , Peng Xu 1, 2 , Biao Yu 1, 2
Affiliation
|
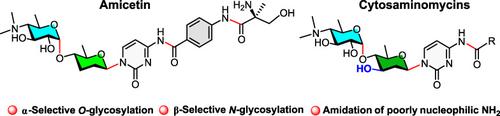
Amicetin and congeners constitute a small family of complex pyrimidine nucleosides, which exhibit strong antibiotic activities against Gram-positive bacteria and notably against strains of Mycobacterium tuberculosis. Herein, we report chemical synthesis of a series of disaccharide congeners of the amicetin family, including amicetin, plicacetin, and cytosaminomycin A—D. It is the first time for successful synthesis of amicetin, the prototypical member, and cytosaminomycins. The synthetic approach employs glycosyl N-phenyltrifluoroacetimidate and thioglycoside donors to construct the characteristic aminodeoxydisaccharides consisting of α-(1→4)-glycosidic linkage, uses gold(I)-catalyzed N-glycosylation to furnish 2-deoxy-β-nucleosides, and finally exploits amidation and global deprotection to complete the syntheses. It is noteworthy that the 3-O-protecting group in the 2-deoxydisaccharide donors is found to be crucial for a successful N-glycosylation to assemble the cytosaminomycin disaccharide nucleosides. 
中文翻译:

核苷类抗生素 Amicetin、Plicacetin 和 Cytosaminomycin A-D 的全合成
Amicetin 和同源物构成了一个复杂的嘧啶核苷的小家族,它们对革兰氏阳性细菌和特别是对结核分枝杆菌菌株表现出很强的抗生素活性。在此,我们报告了一系列阿米西汀家族的双糖同源物的化学合成,包括阿米西汀、普利西汀和胞质霉素 A-D。这是首次成功合成阿米西汀(原型成员)和胞质霉素。合成方法采用糖基N-苯基三氟乙酰亚胺酯和硫糖苷供体构建由 α-(1→4)-糖苷键组成的特征氨基脱氧二糖,使用金 (I)-催化N-糖基化以提供 2-脱氧-β-核苷,最后利用酰胺化和全局脱保护来完成合成。值得注意的是,发现 2-脱氧二糖供体中的 3- O-保护基团对于成功进行N-糖基化以组装胞质霉素双糖核苷至关重要。
更新日期:2021-06-07

中文翻译:

核苷类抗生素 Amicetin、Plicacetin 和 Cytosaminomycin A-D 的全合成
Amicetin 和同源物构成了一个复杂的嘧啶核苷的小家族,它们对革兰氏阳性细菌和特别是对结核分枝杆菌菌株表现出很强的抗生素活性。在此,我们报告了一系列阿米西汀家族的双糖同源物的化学合成,包括阿米西汀、普利西汀和胞质霉素 A-D。这是首次成功合成阿米西汀(原型成员)和胞质霉素。合成方法采用糖基N-苯基三氟乙酰亚胺酯和硫糖苷供体构建由 α-(1→4)-糖苷键组成的特征氨基脱氧二糖,使用金 (I)-催化N-糖基化以提供 2-脱氧-β-核苷,最后利用酰胺化和全局脱保护来完成合成。值得注意的是,发现 2-脱氧二糖供体中的 3- O-保护基团对于成功进行N-糖基化以组装胞质霉素双糖核苷至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号