当前位置:
X-MOL 学术
›
Artif. Organs
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ferrous hemoglobin and hemoglobin-based oxygen carriers acting as a peroxidase can inhibit oxidative damage to endothelial cells caused by hydrogen peroxide
Artificial Organs ( IF 2.2 ) Pub Date : 2021-06-08 , DOI: 10.1111/aor.14009 Shuangshuang Huo 1 , Xiaofeng Lei 1 , Dan He 1 , Hua Zhang 2 , Zhipeng Yang 1 , Wenhua Mu 1 , Ke Fang 1 , Dan Xue 1 , He Li 1 , Xiaoyan Li 1 , Nan Jia 1 , Hongli Zhu 1 , Chao Chen 1 , Kunping Yan 1
Artificial Organs ( IF 2.2 ) Pub Date : 2021-06-08 , DOI: 10.1111/aor.14009 Shuangshuang Huo 1 , Xiaofeng Lei 1 , Dan He 1 , Hua Zhang 2 , Zhipeng Yang 1 , Wenhua Mu 1 , Ke Fang 1 , Dan Xue 1 , He Li 1 , Xiaoyan Li 1 , Nan Jia 1 , Hongli Zhu 1 , Chao Chen 1 , Kunping Yan 1
Affiliation
|
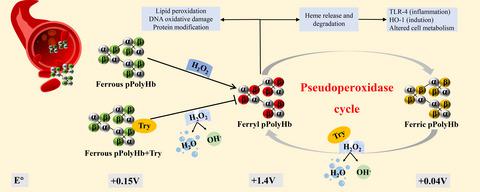
Oxidative damage caused by the ferryl hemoglobin is one of the major clinical adverse reactions of hemoglobin-based oxygen carriers (HBOCs), while the production of reactive oxygen species in a pathological state can oxidize hemoglobin (HbFe2+) to ferryl Hb, which can then enter the pseudoperoxidase cycle, making hemoglobin highly toxic. In this study, we found that ferrous hemoglobin and polymerized porcine hemoglobin (one of the HBOCs) have the peroxidase activity different from the pseudoperoxidase activity of ferric hemoglobin. Ferrous hemoglobin can catalyze the reaction of tyrosine (Tyr) with hydrogen peroxide. In addition, the results also indicated that ferrous hemoglobin and pPolyHb have a strong inhibitory effect on the pseudoperoxidase activity of ferric hemoglobin. Therefore, hydrogen peroxide was consumed in a large amount, which greatly prevented hemoglobin from becoming oxidized and entering the pseudoperoxidase cycle, thus inhibiting ferryl Hb toxicity. We further cultured human umbilical vein endothelial cells and monitored cell morphology, viability, cell cycle, apoptosis, lactate dehydrogenase (LDH) release, and malondialdehydes (MDAs) formation when incubated with H2O2, Tyr, and HbFe2+. HbFe2+ and pPolyHb reduced cell cycle arrest, apoptosis, LDH release, and MDA formation. These results showed that reducing oxidative damage induced by H2O2 and converted hemoglobin from a molecule that is toxic to one that inhibits oxidative damage, suggesting a new strategy for development of a safer HBOCs.
中文翻译:

亚铁血红蛋白和作为过氧化物酶的基于血红蛋白的氧载体可以抑制过氧化氢对内皮细胞的氧化损伤
渡轮血红蛋白引起的氧化损伤是血红蛋白基氧载体(HBOCs)的主要临床不良反应之一,而病理状态下活性氧的产生可氧化血红蛋白(HbFe 2+) 转移到 Hb,然后进入假过氧化物酶循环,使血红蛋白具有剧毒。在这项研究中,我们发现亚铁血红蛋白和聚合猪血红蛋白(HBOCs 之一)具有不同于铁血红蛋白的假过氧化物酶活性的过氧化物酶活性。亚铁血红蛋白可以催化酪氨酸(Tyr)与过氧化氢的反应。此外,结果还表明亚铁血红蛋白和pPolyHb对铁血红蛋白的假过氧化物酶活性有很强的抑制作用。因此,大量消耗过氧化氢,极大地阻止了血红蛋白被氧化进入假过氧化物酶循环,从而抑制了ferryl Hb毒性。我们进一步培养了人脐静脉内皮细胞并监测了细胞形态、活力、细胞周期、2 O 2、Tyr 和 HbFe 2+。HbFe 2+和 pPolyHb 可减少细胞周期停滞、细胞凋亡、LDH 释放和 MDA 形成。这些结果表明,减少由 H 2 O 2诱导的氧化损伤,并将血红蛋白从一种有毒的分子转化为抑制氧化损伤的分子,这表明了一种开发更安全的 HBOC 的新策略。
更新日期:2021-06-08
中文翻译:

亚铁血红蛋白和作为过氧化物酶的基于血红蛋白的氧载体可以抑制过氧化氢对内皮细胞的氧化损伤
渡轮血红蛋白引起的氧化损伤是血红蛋白基氧载体(HBOCs)的主要临床不良反应之一,而病理状态下活性氧的产生可氧化血红蛋白(HbFe 2+) 转移到 Hb,然后进入假过氧化物酶循环,使血红蛋白具有剧毒。在这项研究中,我们发现亚铁血红蛋白和聚合猪血红蛋白(HBOCs 之一)具有不同于铁血红蛋白的假过氧化物酶活性的过氧化物酶活性。亚铁血红蛋白可以催化酪氨酸(Tyr)与过氧化氢的反应。此外,结果还表明亚铁血红蛋白和pPolyHb对铁血红蛋白的假过氧化物酶活性有很强的抑制作用。因此,大量消耗过氧化氢,极大地阻止了血红蛋白被氧化进入假过氧化物酶循环,从而抑制了ferryl Hb毒性。我们进一步培养了人脐静脉内皮细胞并监测了细胞形态、活力、细胞周期、2 O 2、Tyr 和 HbFe 2+。HbFe 2+和 pPolyHb 可减少细胞周期停滞、细胞凋亡、LDH 释放和 MDA 形成。这些结果表明,减少由 H 2 O 2诱导的氧化损伤,并将血红蛋白从一种有毒的分子转化为抑制氧化损伤的分子,这表明了一种开发更安全的 HBOC 的新策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号