当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Photoinduced LMCT: A 1° C–H Abstraction Enables Skeletal Rearrangements and C(sp3)–H Alkylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-08 , DOI: 10.1021/acscatal.1c02285
Yi Cheng Kang 1 , Sean M Treacy 1 , Tomislav Rovis 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-06-08 , DOI: 10.1021/acscatal.1c02285
Yi Cheng Kang 1 , Sean M Treacy 1 , Tomislav Rovis 1
Affiliation
|
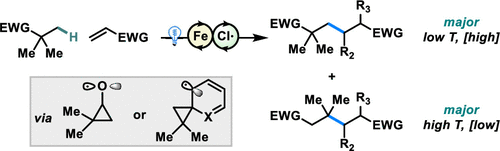
Herein we disclose an iron-catalyzed method to access skeletal rearrangement reactions akin to the Dowd–Beckwith ring expansion from unactivated C(sp3)–H bonds. Photoinduced ligand-to-metal charge transfer at the iron center generates a chlorine radical, which abstracts electron-rich C(sp3)–H bonds. The resulting unstable alkyl radicals can undergo rearrangement in the presence of suitable functionality. Addition to an electron deficient olefin, recombination with a photoreduced iron complex, and subsequent protodemetalation allow for redox-neutral alkylation of the resulting radical. Simple adjustments to the reaction conditions enable the selective synthesis of the directly alkylated or the rearranged-alkylated products. As a radical clock, these rearrangements also enable the measurement of rate constants of addition into various electron deficient olefins in the Giese reaction.
中文翻译:

铁催化光诱导 LMCT:1° C-H 抽象使骨架重排和 C(sp3)-H 烷基化成为可能
在这里,我们公开了一种铁催化的方法来进行骨架重排反应,类似于未活化的 C(sp 3 )-H 键的 Dowd-Beckwith 环扩展。铁中心的光诱导配体-金属电荷转移产生氯自由基,从而提取富电子 C(sp 3)–H 键。产生的不稳定烷基自由基可以在合适的官能团存在下进行重排。添加到缺电子烯烃中,与光还原的铁络合物复合,以及随后的原型去金属化,可实现所得自由基的氧化还原中性烷基化。对反应条件的简单调整能够选择性地合成直接烷基化或重排烷基化产物。作为一个激进的时钟,这些重排还可以测量在吉斯反应中添加到各种缺电子烯烃中的速率常数。
更新日期:2021-06-18
中文翻译:

铁催化光诱导 LMCT:1° C-H 抽象使骨架重排和 C(sp3)-H 烷基化成为可能
在这里,我们公开了一种铁催化的方法来进行骨架重排反应,类似于未活化的 C(sp 3 )-H 键的 Dowd-Beckwith 环扩展。铁中心的光诱导配体-金属电荷转移产生氯自由基,从而提取富电子 C(sp 3)–H 键。产生的不稳定烷基自由基可以在合适的官能团存在下进行重排。添加到缺电子烯烃中,与光还原的铁络合物复合,以及随后的原型去金属化,可实现所得自由基的氧化还原中性烷基化。对反应条件的简单调整能够选择性地合成直接烷基化或重排烷基化产物。作为一个激进的时钟,这些重排还可以测量在吉斯反应中添加到各种缺电子烯烃中的速率常数。































 京公网安备 11010802027423号
京公网安备 11010802027423号