当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifonazole dissolved in numerous aqueous alcohol mixtures: Solvent effect, enthalpy–entropy compensation, extended Hildebrand solubility parameter approach and preferential solvation
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-06-08 , DOI: 10.1016/j.molliq.2021.116671 Wanxin Li , Jiayi Yuan , Xinling Wang , Weizhong Shi , Hongkun Zhao , Rong Xing , Abolghasem Jouyban , William E. Acree
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-06-08 , DOI: 10.1016/j.molliq.2021.116671 Wanxin Li , Jiayi Yuan , Xinling Wang , Weizhong Shi , Hongkun Zhao , Rong Xing , Abolghasem Jouyban , William E. Acree
|
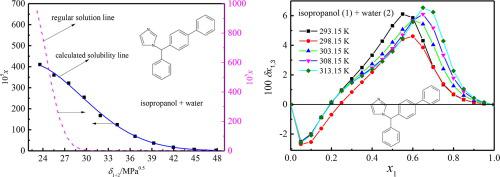
The accessible mole fraction solubility of bifonazole in n -propanol/methanol/isopropanol/propylene glycol (PG) (1) + water (2) mixtures at 298.15 K was investigated in the light of molecular interactions between solvent and solute via extended Hildebrand solubility approach (EHSA). For aqueous methanol/PG systems within entire mole fraction range of methanol/PG, aqueous n -propanol system within mole fraction range of n -propanol from 0 to 0.8 and aqueous isopropanol system within mole fraction range of isopropanol from 0 to 0.7, the solute–solvent interaction energies are larger than the ones of geometric mean for regular solution, asserting that there exists some association between bifonazole and mixtures; whereas within the other composition regions for the aqueous isopropanol/n -propanol systems, the solutions consisting of bifonazole present regular behavior at several solubility points. The inverse Kirkwood–Buff integrals tool was utilized to investigate the local mole fractions of bifonazole in the solutions of alcohol (1) + water (2) in the light of the solubility. For the aqueous solutions of isopropanol/n -propanol with middle and isopropanol/n -propanol-rich mole fractions, preferential solvation of bifonazole was observed for isopropanol/n -propanol. Bifonazole was not solvated preferentially by PG/methanol in the above composition regions for aqueous PG/methanol mixtures. The solvent effect was quantitatively described by modeling the solubility change by means of the linear solvation energy relationships. The solubility parameter and dipolarity-polarizability of system descriptors presented the dominant contributions to variation of solubility values. The transfer and dissolution properties, e.g. entropy, Gibbs free energy change and enthalpy were calculated. In addition, the analysis of enthalpy–entropy compensation was performed, specifying that the variation of bifonazole solubility in the four aqueous alcohol solutions was controlled by two different mechanisms, enthalpy-driven and entropy-driven.
中文翻译:

联苯苄唑溶于多种醇类混合物中:溶剂效应、焓-熵补偿、扩展的 Hildebrand 溶解度参数方法和优先溶剂化
根据溶剂和溶质之间的分子相互作用,通过扩展 Hildebrand 溶解度方法 (EHSA) 研究了联苯苄唑在 298.15 K 下正丙醇/甲醇/异丙醇/丙二醇 (PG) (1) + 水 (2) 混合物中可溶的可溶摩尔分数溶解度。对于甲醇/PG整个摩尔级分范围内的甲醇/PG水溶液体系,正丙醇摩尔级分范围为0至0.8的正丙醇水溶液体系和异丙醇摩尔级分范围为0至0.7的异丙醇水溶液系,溶质-溶剂相互作用能大于常规溶液的几何平均值, 断言联苯苋唑和混合物之间存在某种关联;而在异丙醇/正丙醇水溶液体系的其他组成区域内,由联苯苄唑组成的溶液在多个溶解度点上表现出规则的行为。根据溶解度,使用逆 Kirkwood-Buff 积分工具研究氢萧唑在醇 (1) + 水 (2) 溶液中的局部摩尔分数。对于具有中间和富含异丙醇/n-丙醇的异丙醇/正丙醇的水溶液,观察到异丙醇/正丙醇对联苯苄唑的优先溶剂化。对于PG /甲醇水溶液混合物,在上述组成区域中,联苯苄唑未优先被 PG /甲醇溶剂化。通过线性溶剂化能关系对溶解度变化进行建模,定量描述溶剂效应。系统描述符的溶解度参数和偶极极化率代表了对溶解度值变化的主要贡献。计算了转移和溶解性质,例如熵、吉布斯自由能变化和焓。 此外,还进行了焓-熵补偿分析,指出联苯苄唑在四种醇水溶液中溶解度的变化受焓驱动和熵驱动两种不同机制控制。
更新日期:2021-06-08
中文翻译:

联苯苄唑溶于多种醇类混合物中:溶剂效应、焓-熵补偿、扩展的 Hildebrand 溶解度参数方法和优先溶剂化
根据溶剂和溶质之间的分子相互作用,通过扩展 Hildebrand 溶解度方法 (EHSA) 研究了联苯苄唑在 298.15 K 下正丙醇/甲醇/异丙醇/丙二醇 (PG) (1) + 水 (2) 混合物中可溶的可溶摩尔分数溶解度。对于甲醇/PG整个摩尔级分范围内的甲醇/PG水溶液体系,正丙醇摩尔级分范围为0至0.8的正丙醇水溶液体系和异丙醇摩尔级分范围为0至0.7的异丙醇水溶液系,溶质-溶剂相互作用能大于常规溶液的几何平均值, 断言联苯苋唑和混合物之间存在某种关联;而在异丙醇/正丙醇水溶液体系的其他组成区域内,由联苯苄唑组成的溶液在多个溶解度点上表现出规则的行为。根据溶解度,使用逆 Kirkwood-Buff 积分工具研究氢萧唑在醇 (1) + 水 (2) 溶液中的局部摩尔分数。对于具有中间和富含异丙醇/n-丙醇的异丙醇/正丙醇的水溶液,观察到异丙醇/正丙醇对联苯苄唑的优先溶剂化。对于PG /甲醇水溶液混合物,在上述组成区域中,联苯苄唑未优先被 PG /甲醇溶剂化。通过线性溶剂化能关系对溶解度变化进行建模,定量描述溶剂效应。系统描述符的溶解度参数和偶极极化率代表了对溶解度值变化的主要贡献。计算了转移和溶解性质,例如熵、吉布斯自由能变化和焓。 此外,还进行了焓-熵补偿分析,指出联苯苄唑在四种醇水溶液中溶解度的变化受焓驱动和熵驱动两种不同机制控制。































 京公网安备 11010802027423号
京公网安备 11010802027423号