Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2021-06-08 , DOI: 10.1016/j.jelechem.2021.115450
Nian Xiao , Liang Wang , Hongying Wang , Sufang Wang , Tao Zhu
|
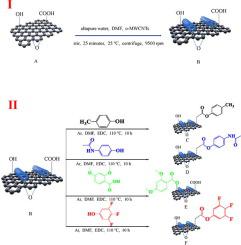
通过Aldol反应合成了四种新型吸电子/供电子基团-氧化石墨烯-氧化多壁碳纳米管(EWG/EDG-GO-o-MWCNTs)纳米复合材料。将 EWG 和 EDG 成功接枝到 GO-o-MWCNTs 上,通过傅里叶变换红外光谱 (FT-IR) 和元素分析 (EDX) 对其进行了研究。通过电化学测试和静态吸附实验研究了EWG和EDG对GOπ体系尺寸的影响。EDG-GO-o-MWCNTs的电荷转移电阻(RCT)显着小于电化学阻抗谱(EIS)的EWG-GO-o-MWCNTs,这表明EDG增强了GO的π系统的电子密度,增加了GO 的电子密度,提高了 GO 的电子转移速率。EWG消除了GO的π系统的电子密度,这导致电子转移减慢。静态吸附表明呋塞米具有不同的吸附容量 (2, 4DMOA-GO-o-MWCNTs (26.16 μg/mL) > 3, 4, 5TFPh-GO-o-MWCNTs (22.61 μg/mL) > 4MPh-GO-o -MWCNTs (18.30 μg/mL) > 4AcPh-GO-o-MWCNTs (10.66 μg/mL) > GO (4.39 μg/mL))。结果表明,EWG/EDG-GO-o-MWCNTs 纳米复合材料可以通过氢键和 π-π 相互作用轻松吸附呋塞米。电化学测试和静态吸附实验表明,EWG和EDG可以改变GO的π体系。39 μg/mL))。结果表明,EWG/EDG-GO-o-MWCNTs 纳米复合材料可以通过氢键和 π-π 相互作用轻松吸附呋塞米。电化学测试和静态吸附实验表明,EWG和EDG可以改变GO的π体系。39 μg/mL))。结果表明,EWG/EDG-GO-o-MWCNTs 纳米复合材料可以通过氢键和 π-π 相互作用轻松吸附呋塞米。电化学测试和静态吸附实验表明,EWG和EDG可以改变GO的π体系。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号