当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Turnover and Inactivation Mechanisms for (S)-3-Amino-4,4-difluorocyclopent-1-enecarboxylic Acid, a Selective Mechanism-Based Inactivator of Human Ornithine Aminotransferase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-07 , DOI: 10.1021/jacs.1c02456 Sida Shen 1 , Arseniy Butrin 2 , Peter F Doubleday 3 , Rafael D Melani 3 , Brett A Beaupre 2 , Mauricio T Tavares 4 , Glaucio M Ferreira 5 , Neil L Kelleher 1, 3 , Graham R Moran 2 , Dali Liu 2 , Richard B Silverman 1, 3, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-06-07 , DOI: 10.1021/jacs.1c02456 Sida Shen 1 , Arseniy Butrin 2 , Peter F Doubleday 3 , Rafael D Melani 3 , Brett A Beaupre 2 , Mauricio T Tavares 4 , Glaucio M Ferreira 5 , Neil L Kelleher 1, 3 , Graham R Moran 2 , Dali Liu 2 , Richard B Silverman 1, 3, 6
Affiliation
|
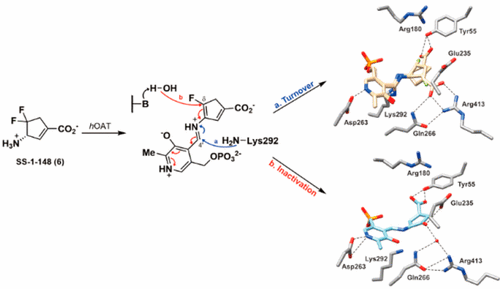
The inhibition of human ornithine δ-aminotransferase (hOAT) is a potential therapeutic approach to treat hepatocellular carcinoma. In this work, (S)-3-amino-4,4-difluorocyclopent-1-enecarboxylic acid (SS-1-148, 6) was identified as a potent mechanism-based inactivator of hOAT while showing excellent selectivity over other related aminotransferases (e.g., GABA-AT). An integrated mechanistic study was performed to investigate the turnover and inactivation mechanisms of 6. A monofluorinated ketone (M10) was identified as the primary metabolite of 6 in hOAT. By soaking hOAT holoenzyme crystals with 6, a precursor to M10 was successfully captured. This gem-diamine intermediate, covalently bound to Lys292, observed for the first time in hOAT/ligand crystals, validates the turnover mechanism proposed for 6. Co-crystallization yielded hOAT in complex with 6 and revealed a novel noncovalent inactivation mechanism in hOAT. Native protein mass spectrometry was utilized for the first time in a study of an aminotransferase inactivator to validate the noncovalent interactions between the ligand and the enzyme; a covalently bonded complex was also identified as a minor form observed in the denaturing intact protein mass spectrum. Spectral and stopped-flow kinetic experiments supported a lysine-assisted E2 fluoride ion elimination, which has never been observed experimentally in other studies of related aminotransferase inactivators. This elimination generated the second external aldimine directly from the initial external aldimine, rather than the typical E1cB elimination mechanism, forming a quinonoid transient state between the two external aldimines. The use of native protein mass spectrometry, X-ray crystallography employing both soaking and co-crystallization methods, and stopped-flow kinetics allowed for the detailed elucidation of unusual turnover and inactivation pathways.
中文翻译:

(S)-3-Amino-4,4-difluorocyclopent-1-enecarboxylic Acid 的转换和失活机制,一种基于选择性机制的人鸟氨酸转氨酶灭活剂
抑制人鸟氨酸 δ-氨基转移酶 ( h OAT) 是治疗肝细胞癌的潜在治疗方法。在这项工作中,( S )-3-amino-4,4-difluorocyclopent-1-enecarboxylic acid (SS-1-148, 6 ) 被确定为一种有效的基于机制的h OAT灭活剂,同时显示出优于其他相关的选择性氨基转移酶(例如,GABA-AT)。进行了综合机理研究以研究6的周转和失活机制。单氟化酮 ( M10 ) 被确定为6 in h OAT 的主要代谢物。通过将h OAT 全酶晶体与如图6所示,成功捕获了M10的前体。这种与 Lys292 共价结合的宝石-二胺中间体首次在h OAT/配体晶体中观察到,验证了为6提出的周转机制。共结晶产生与6复合的h OAT,并揭示了 h 中一种新的非共价失活机制燕麦。天然蛋白质质谱法首次用于氨基转移酶灭活剂的研究,以验证配体和酶之间的非共价相互作用;共价结合的复合物也被鉴定为在变性完整蛋白质质谱中观察到的次要形式。光谱和停流动力学实验支持赖氨酸辅助的 E2 氟离子消除,这在相关转氨酶灭活剂的其他研究中从未通过实验观察到。这种消除直接从最初的外部醛亚胺生成第二个外部醛亚胺,而不是典型的 E1cB 消除机制,在两个外部醛亚胺之间形成醌类瞬态。使用天然蛋白质质谱法,
更新日期:2021-06-17
中文翻译:

(S)-3-Amino-4,4-difluorocyclopent-1-enecarboxylic Acid 的转换和失活机制,一种基于选择性机制的人鸟氨酸转氨酶灭活剂
抑制人鸟氨酸 δ-氨基转移酶 ( h OAT) 是治疗肝细胞癌的潜在治疗方法。在这项工作中,( S )-3-amino-4,4-difluorocyclopent-1-enecarboxylic acid (SS-1-148, 6 ) 被确定为一种有效的基于机制的h OAT灭活剂,同时显示出优于其他相关的选择性氨基转移酶(例如,GABA-AT)。进行了综合机理研究以研究6的周转和失活机制。单氟化酮 ( M10 ) 被确定为6 in h OAT 的主要代谢物。通过将h OAT 全酶晶体与如图6所示,成功捕获了M10的前体。这种与 Lys292 共价结合的宝石-二胺中间体首次在h OAT/配体晶体中观察到,验证了为6提出的周转机制。共结晶产生与6复合的h OAT,并揭示了 h 中一种新的非共价失活机制燕麦。天然蛋白质质谱法首次用于氨基转移酶灭活剂的研究,以验证配体和酶之间的非共价相互作用;共价结合的复合物也被鉴定为在变性完整蛋白质质谱中观察到的次要形式。光谱和停流动力学实验支持赖氨酸辅助的 E2 氟离子消除,这在相关转氨酶灭活剂的其他研究中从未通过实验观察到。这种消除直接从最初的外部醛亚胺生成第二个外部醛亚胺,而不是典型的 E1cB 消除机制,在两个外部醛亚胺之间形成醌类瞬态。使用天然蛋白质质谱法,






























 京公网安备 11010802027423号
京公网安备 11010802027423号