当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Stereoselective Synthesis of (1R,4R)- and (1S,4S)-2-Oxa-5-azabicyclo[2.2.2]octane
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-06-07 , DOI: 10.1021/acs.oprd.1c00098 Matthew L. Maddess 1 , Ed Cleator 2 , Mariko Morimoto 1 , Adrian Goodyear 2 , Alejandro Dieguez-Vazquez 2 , Andrew Gibb 2 , Andy Kirtley 2 , Jie Wang 3 , Ji Qi 4 , Lingzhu Kong 3 , Mahbub Alam 2 , Stephen Keen 2 , Steven F. Oliver 2 , Xin Wen 1 , Yu-Hong Lam 5
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-06-07 , DOI: 10.1021/acs.oprd.1c00098 Matthew L. Maddess 1 , Ed Cleator 2 , Mariko Morimoto 1 , Adrian Goodyear 2 , Alejandro Dieguez-Vazquez 2 , Andrew Gibb 2 , Andy Kirtley 2 , Jie Wang 3 , Ji Qi 4 , Lingzhu Kong 3 , Mahbub Alam 2 , Stephen Keen 2 , Steven F. Oliver 2 , Xin Wen 1 , Yu-Hong Lam 5
Affiliation
|
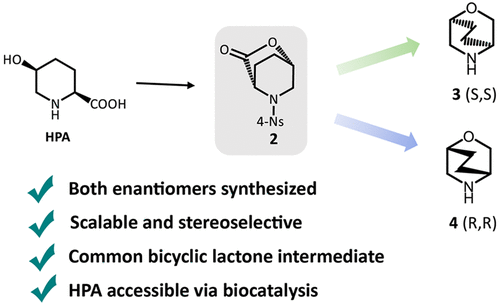
Despite the prevalence of morpholine derivatives and bridged heterocycles in medicinally relevant compounds, bridged bicyclic morpholines remain scarce because of the challenges associated with their synthesis. MRK A, an IDH1mut inhibitor for the treatment of glioma, derives its potency in part from substitution of a zigzag 2,5-bicyclic morpholine, 2-oxa-5-azabicyclo[2.2.2]octane, at C8. While existing entries suffered from low yields and lack of stereochemical control, we developed concise stereospecific routes toward both enantiomers of the zigzag morpholine antipode. The key common intermediate in the two routes was a chiral bicyclic lactone, which was readily synthesized following our previous synthesis of relebactam from optically pure (2S,5S)-5-hydroxypiperidine-2-carboxylic acid (HPA). The desired (R,R) enantiomer for incorporation into MRK A required inversion of both stereocenters of the bicyclic lactone intermediate, which was accomplished by epimerization via a crystallization-induced diastereomer transformation process followed by a key Ti(OiPr)4-mediated intramolecular SN2 ring closure. By this method, the (R,R)-zigzag morpholine was synthesized in six steps from HPA in 25% overall yield.
中文翻译:

(1R,4R)-和(1S,4S)-2-Oxa-5-氮杂双环[2.2.2]辛烷的立体选择性合成的发展
尽管在药用相关化合物中普遍存在吗啉衍生物和桥接杂环,但由于与合成相关的挑战,桥接双环吗啉仍然稀缺。MRK A 是一种用于治疗神经胶质瘤的 IDH1 mut抑制剂,其效力部分来自于在 C8 处取代锯齿形 2,5-双环吗啉、2-oxa-5-azabicyclo[2.2.2]octane。虽然现有条目的产量低且缺乏立体化学控制,但我们针对之字形吗啉对映体的两种对映异构体开发了简洁的立体特异性路线。两条路线中的关键共同中间体是手性双环内酯,在我们之前从光学纯 (2 S ,5 S)-5-羟基哌啶-2-羧酸 (HPA)。所需的 ( R , R ) 对映异构体用于掺入 MRK A 需要反转双环内酯中间体的两个立体中心,这是通过结晶诱导的非对映异构体转化过程差向异构化,然后是关键的 Ti(O i Pr) 4介导的分子内 S N 2 闭环。通过这种方法,( R , R )-之字形吗啉由 HPA 分六步合成,总产率为 25%。
更新日期:2021-06-07
中文翻译:

(1R,4R)-和(1S,4S)-2-Oxa-5-氮杂双环[2.2.2]辛烷的立体选择性合成的发展
尽管在药用相关化合物中普遍存在吗啉衍生物和桥接杂环,但由于与合成相关的挑战,桥接双环吗啉仍然稀缺。MRK A 是一种用于治疗神经胶质瘤的 IDH1 mut抑制剂,其效力部分来自于在 C8 处取代锯齿形 2,5-双环吗啉、2-oxa-5-azabicyclo[2.2.2]octane。虽然现有条目的产量低且缺乏立体化学控制,但我们针对之字形吗啉对映体的两种对映异构体开发了简洁的立体特异性路线。两条路线中的关键共同中间体是手性双环内酯,在我们之前从光学纯 (2 S ,5 S)-5-羟基哌啶-2-羧酸 (HPA)。所需的 ( R , R ) 对映异构体用于掺入 MRK A 需要反转双环内酯中间体的两个立体中心,这是通过结晶诱导的非对映异构体转化过程差向异构化,然后是关键的 Ti(O i Pr) 4介导的分子内 S N 2 闭环。通过这种方法,( R , R )-之字形吗啉由 HPA 分六步合成,总产率为 25%。







































 京公网安备 11010802027423号
京公网安备 11010802027423号