当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glutathione-Activated NO-/ROS-Generation Nanoparticles to Modulate the Tumor Hypoxic Microenvironment for Enhancing the Effect of HIFU-Combined Chemotherapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-06-04 , DOI: 10.1021/acsami.1c07494 Qianyan Li 1 , Jingni Zhang 1 , Jingnan Li 1 , Hemin Ye 1 , Meixuan Li 1 , Wei Hou 1 , Huanan Li 1 , Zhibiao Wang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-06-04 , DOI: 10.1021/acsami.1c07494 Qianyan Li 1 , Jingni Zhang 1 , Jingnan Li 1 , Hemin Ye 1 , Meixuan Li 1 , Wei Hou 1 , Huanan Li 1 , Zhibiao Wang 1
Affiliation
|
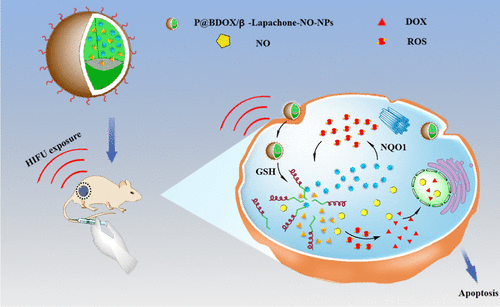
The combination of high-intensity focused ultrasound (HIFU) and chemotherapy has promising potential in the synergistic treatment of various types of solid tumors. However, the clinical efficacy of HIFU in combination chemotherapy is often impeded by the pre-existing hypoxia tumor microenvironment-induced multidrug resistance (MDR). Therefore, it is imperative for HIFU combined with chemotherapy to overcome MDR by improving the tumor hypoxic microenvironment. Hence, we developed highly stable nanoparticles (P@BDOX/β-lapachone-NO-NPs) with intracellular nitric oxide (NO)- and reactive oxygen species (ROS)-generating capabilities at the tumor site to relieve the hypoxic tumor microenvironment in solid tumors. Doxorubicin prodrug (boronate-DOX, BDOX) and β-lapachone were concurrently loaded onto actively targeted pH (low) insertion peptides (pHLIPs)-poly(ethylene glycol) and nitrated gluconic acid copolymers. Our results showed that the ability of P@BDOX/β-lapachone-NO-NPs to generate NO and ROS simultaneously is vital for the sensitization of hypoxic solid tumors for chemotherapy, as evidenced by the suppression of tumor cells and tissues (in vitro and in the nude mice model). Thus, this combined therapy holds considerable potential in the management of hypoxic solid tumors.
中文翻译:

谷胱甘肽激活 NO/ROS 生成纳米粒子调节肿瘤缺氧微环境以增强 HIFU 联合化疗的效果
高强度聚焦超声(HIFU)与化疗的结合在多种类型实体瘤的协同治疗中具有广阔的前景。然而,HIFU在联合化疗中的临床疗效往往受到先前存在的缺氧肿瘤微环境诱导的多药耐药性(MDR)的阻碍。因此,HIFU联合化疗通过改善肿瘤缺氧微环境来克服MDR势在必行。因此,我们开发了高度稳定的纳米粒子(P@BDOX/β-lapachone-NO-NPs),具有在肿瘤部位产生细胞内一氧化氮(NO)和活性氧(ROS)的能力,以缓解固体中缺氧的肿瘤微环境。肿瘤。将阿霉素前药(硼酸-DOX、BDOX)和 β-拉帕酮同时负载到主动靶向 pH(低)插入肽 (pHLIP)-聚乙二醇和硝化葡萄糖酸共聚物上。我们的结果表明,P@BDOX/β-lapachone-NO-NPs 同时产生 NO 和 ROS 的能力对于缺氧实体瘤对化疗的敏感性至关重要,这一点通过对肿瘤细胞和组织的抑制(体外和体内)证明了这一点。在裸鼠模型中)。因此,这种联合疗法在缺氧实体瘤的治疗中具有相当大的潜力。
更新日期:2021-06-16
中文翻译:

谷胱甘肽激活 NO/ROS 生成纳米粒子调节肿瘤缺氧微环境以增强 HIFU 联合化疗的效果
高强度聚焦超声(HIFU)与化疗的结合在多种类型实体瘤的协同治疗中具有广阔的前景。然而,HIFU在联合化疗中的临床疗效往往受到先前存在的缺氧肿瘤微环境诱导的多药耐药性(MDR)的阻碍。因此,HIFU联合化疗通过改善肿瘤缺氧微环境来克服MDR势在必行。因此,我们开发了高度稳定的纳米粒子(P@BDOX/β-lapachone-NO-NPs),具有在肿瘤部位产生细胞内一氧化氮(NO)和活性氧(ROS)的能力,以缓解固体中缺氧的肿瘤微环境。肿瘤。将阿霉素前药(硼酸-DOX、BDOX)和 β-拉帕酮同时负载到主动靶向 pH(低)插入肽 (pHLIP)-聚乙二醇和硝化葡萄糖酸共聚物上。我们的结果表明,P@BDOX/β-lapachone-NO-NPs 同时产生 NO 和 ROS 的能力对于缺氧实体瘤对化疗的敏感性至关重要,这一点通过对肿瘤细胞和组织的抑制(体外和体内)证明了这一点。在裸鼠模型中)。因此,这种联合疗法在缺氧实体瘤的治疗中具有相当大的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号