European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-06-05 , DOI: 10.1016/j.ejmech.2021.113592 Shuni Wang 1 , Hong Yang 1 , Mingbo Su 2 , Fulin Lian 1 , Zhanqing Cong 1 , Rongrui Wei 1 , Yubo Zhou 3 , Xingjun Li 1 , Xingling Zheng 1 , Chunpu Li 4 , Xuhong Fu 1 , Xu Han 1 , Qiongyu Shi 1 , Cong Li 1 , Naixia Zhang 1 , Meiyu Geng 1 , Hong Liu 5 , Jia Li 6 , Xun Huang 4 , Jiang Wang 4
|
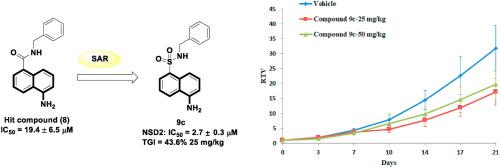
Approximately 20% of multiple myeloma (MM) are caused by a chromosomal translocation t (4;14) that leads to the overexpression of the nuclear receptor binding SET domain-protein 2 (NSD2) histone methyltransferase. NSD2 catalyzes the methylation of lysine 36 on histone H3 (H3K36me2) and is associated with transcriptionally active regions. Using high-throughput screening (HTS) with biological analyses, a series of 5-aminonaphthalene derivatives were designed and synthesized as novel NSD2 inhibitors. Among all the prepared compounds, 9c displayed a good NSD2 inhibitory activity (IC50 = 2.7 μM) and selectivity against both SET-domain-containing and non-SET-domain-containing methyltransferases. Preliminary research indicates the inhibition mechanism of compound 9c by significantly suppressed the methylation of H3K36me2. Compound 9c specifically inhibits the proliferation of the human B cell precursor leukemia cell line RS4:11 and the human myeloma cell line KMS11 by inducing cell cycle arrest and apoptosis with little cytotoxicity. It has been reported that the anti-cancer effect of compound 9c is partly achieved by completely suppressing the transcriptional activation of NSD2-targeted genes. When administered intraperitoneally at 25 mg/kg, compound 9c suppressed the tumor growth of RS4:11 xenografts in vivo and no body weight loss was detected in the tested SCID mice.
中文翻译:

5-氨基萘衍生物作为选择性非核苷核受体结合 SET 结构域蛋白 2 (NSD2) 抑制剂,用于治疗多发性骨髓瘤
大约 20% 的多发性骨髓瘤 (MM) 是由染色体易位 t (4;14) 引起的,该易位导致核受体结合 SET 结构域蛋白 2 (NSD2) 组蛋白甲基转移酶的过度表达。NSD2 催化组蛋白 H3 (H3K36me2) 上赖氨酸 36 的甲基化,并与转录活性区域相关。使用高通量筛选 (HTS) 和生物学分析,设计并合成了一系列 5-氨基萘衍生物作为新型 NSD2 抑制剂。在所有制备的化合物中,9c显示出良好的 NSD2 抑制活性 (IC 50 = 2.7 μM) 和对含 SET 域和不含 SET 域的甲基转移酶的选择性。初步研究表明化合物9c的抑制机制通过显着抑制 H3K36me2 的甲基化。化合物9c通过诱导细胞周期停滞和细胞凋亡而特异性抑制人 B 细胞前体白血病细胞系 RS4:11 和人骨髓瘤细胞系 KMS11 的增殖,而细胞毒性很小。据报道,化合物9c的抗癌作用部分是通过完全抑制NSD2靶向基因的转录激活来实现的。当以 25 mg/kg 腹膜内给药时,化合物9c在体内抑制了 RS4:11 异种移植物的肿瘤生长,并且在测试的 SCID 小鼠中未检测到体重减轻。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号