Environmental Pollution ( IF 7.6 ) Pub Date : 2021-06-05 , DOI: 10.1016/j.envpol.2021.117537 Ye Zhang 1 , Fan Ni 2 , Jinsong He 1 , Fei Shen 1 , Shihuai Deng 1 , Dong Tian 1 , Yanzong Zhang 3 , Yan Liu 3 , Chao Chen 1 , Jianmei Zou 1
|
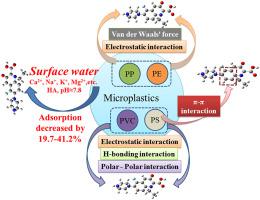
Microplastics (MPs) as carriers of various contaminants have attracted more attentions in water environments. However, the interactions between MPs and norfloxacin (NOR) in natural water environments were still not systematically studied. In this study, the adsorption of NOR onto four typical types of MPs (polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyvinyl chloride (PVC)) was investigated in simulated natural water and real surface water, and the adsorption mechanisms were deeply explored to provide fundamental understandings of the MPs-NOR complicated pollution. The results showed that the kinetics of NOR onto all MPs obeyed pseudo-second-order model, and was greatly slowed down at lower temperature or higher salinity. The intrinsic structure and surface area of MPs played important roles in the adsorption behavior of NOR on these four types of MPs. The adsorption isotherm of NOR onto all MPs could be well described by linear model, with the Kd values following the order of PVC > PS > PE > PP (i.e. 6.229–11.901 L/μg) in simulated natural water. However, in surface water the adsorption isotherms of NOR on all MPs could be well fitted by Freundlich model. For all MPs, the adsorption of NOR was quite pH-dependent due to the electrostatic interactions. Furthermore, the salinity and the presence of natural organic matters (NOM) had significantly hindered the NOR adsorption. More importantly, compared with adsorption behavior in simulated natural water, the competition of coexisting substances such as cations and NOM for adsorption sites and higher water pH dramatically reduced the adsorption of NOR onto all types of MPs in Jiang'an River, with the reduction rate of 19.7–41.2%. Finally, the mechanism studies indicated that the electrostatic attractions played a key role in the adsorptions of NOR onto MPs, and π-π, H-bonding, polar-polar, and Van Der Waals interactions were also involved in adsorption processes.
中文翻译:

模拟天然水和真实地表水中诺氟沙星在微塑料上不同吸附的机理研究
微塑料(MPs)作为各种污染物的载体在水环境中引起了越来越多的关注。然而,MPs 与诺氟沙星(NOR)在天然水环境中的相互作用仍未得到系统研究。在本研究中,在模拟天然水和真实地表水中研究了 NOR 在四种典型类型的 MPs(聚乙烯 (PE)、聚丙烯 (PP)、聚苯乙烯 (PS) 和聚氯乙烯 (PVC))上的吸附,以及深入探索了吸附机制,以提供对 MPs-NOR 复杂污染的基本理解。结果表明,NOR在所有MPs上的动力学遵循伪二级模型,并且在较低温度或较高盐度下大大减慢。MPs 的内在结构和表面积在 NOR 对这四种 MPs 的吸附行为中起着重要作用。NOR 在所有 MPs 上的吸附等温线可以用线性模型很好地描述,其中 Kd模拟天然水中的数值按照 PVC > PS > PE > PP(即 6.229–11.901 L/μg)的顺序排列。然而,在地表水中,所有 MP 上 NOR 的吸附等温线都可以通过 Freundlich 模型很好地拟合。对于所有 MP,由于静电相互作用,NOR 的吸附非常依赖 pH。此外,盐度和天然有机物 (NOM) 的存在显着阻碍了 NOR 的吸附。更重要的是,与模拟天然水中的吸附行为相比,共存物质如阳离子和 NOM 对吸附位点的竞争和较高的水 pH 值显着降低了 NOR 对江安河各类 MPs 的吸附,降低速率为19.7–41.2%。最后,































 京公网安备 11010802027423号
京公网安备 11010802027423号