当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of DNA binding and bioactivities of furan cored Schiff base Cu (II), Ni (II), and Co (III) complexes: Synthesis, characterization and spectroscopic properties
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-06-03 , DOI: 10.1002/aoc.6326 Kadtala Venkateswarlu 1 , Sreenu Daravath 1 , Gali Ramesh 1 , P. V. Anantha Lakshmi 1 , Shivaraj 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-06-03 , DOI: 10.1002/aoc.6326 Kadtala Venkateswarlu 1 , Sreenu Daravath 1 , Gali Ramesh 1 , P. V. Anantha Lakshmi 1 , Shivaraj 1
Affiliation
|
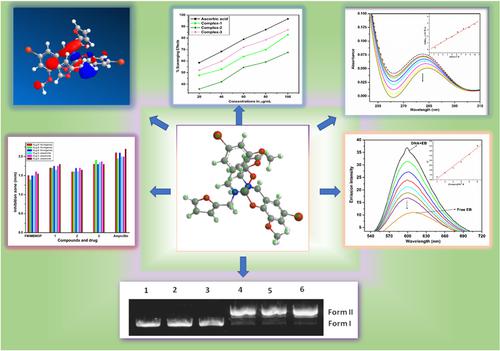
Novel binary metal complexes, 1 [Cu(FMIMBMOP)2], 2 [Ni(FMIMBMOP)2] and 3 [Co(FMIMBMOP)3], where FMIMBMOP (2-((E)-((furan-2-yl)methylimino)methyl)-4-bromo-6-methoxyphenol), were synthesized and characterized using different analytical techniques. Result of spectral studies reveals that square planar geometry is assigned for Cu (II) and Ni (II) complexes, whereas octahedral geometry is assigned for Co (III) complex. The thermodynamic and kinetic parameters for the degradation of the complexes were ascertained by Coats–Redfern method for thermogravimetric data attained from thermogravimetric analysis (TGA). The stability of the complexes has been calculated from quantum chemical parameters using highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energies. The DNA binding of complexes was studied by using ultraviolet–visible (UV–Vis) spectroscopic technique; screening their ability to bind to calf thymus DNA (CT-DNA) showed that the complexes can interact with CT-DNA through intercalation mood, where the Kb values are 2.59 ± 0.01 × 104, 7.43 ± 0.03 × 103, and 6.73 ± 0.02 × 104 M−1 for 1, 2, and 3, respectively. Stern–Volmer quenching constant (Ksv) values ranged from 2.32 ± 0.03 × 103 to 1.66 ± 0.02 × 104 M−1 were calculated for complexes from fluorescence studies. The oxidative and photolytic cleavage of supercoiled pBR322 DNA was studied and found that the complexes have cleaved this DNA effectively. The novel metal complexes have shown significant antioxidant activity against DPPH radical. The antibacterial activity of Schiff base and its metal complexes screened against Bacillus thuringiensis, Streptococcus pneumoniae, Escherichia coli, and Pseudomonas putida was investigated, and the results indicated that the metal complexes have better results than Schiff base ligand. The catalytic ability of metal complexes 1, 2, and 3 was found to be 3 > 1 > 2.
中文翻译:

呋喃核席夫碱 Cu (II)、Ni (II) 和 Co (III) 复合物的 DNA 结合和生物活性研究:合成、表征和光谱特性
新颖二元金属络合物,1 [铜(FMIMBMOP)2 ],2 [镍(FMIMBMOP)2 ]和3 [CO(FMIMBMOP)3 ],其中FMIMBMOP(2 - ((E) - ((˚F URAN -2-基)米乙基我氨基)米乙基)-4- b罗莫-6-米ETH ö XY phenol),使用不同的分析技术合成和表征。光谱研究结果表明,Cu (II) 和 Ni (II) 配合物为方形平面几何形状,而 Co (III) 配合物为八面体几何形状。复合物降解的热力学和动力学参数通过 Coats-Redfern 方法确定,用于从热重分析 (TGA) 获得的热重数据。使用最高占据分子轨道 (HOMO)-最低未占据分子轨道 (LUMO) 能量从量子化学参数计算了配合物的稳定性。使用紫外-可见(UV-Vis)光谱技术研究了复合物的DNA结合;ķ b值是2.59±0.01×10 4,7.43±0.03×10 3,和6.73±0.02×10 4 中号-1为1,2,和3,分别。Stern–Volmer 猝灭常数 ( K sv ) 值范围从 2.32 ± 0.03 × 10 3到 1.66 ± 0.02 × 10 4 M -1计算来自荧光研究的复合物。研究了超螺旋 pBR322 DNA 的氧化和光解裂解,发现复合物有效地裂解了该 DNA。新型金属配合物对 DPPH 自由基显示出显着的抗氧化活性。考察了席夫碱及其金属配合物对苏云金芽孢杆菌、肺炎链球菌、大肠杆菌和恶臭假单胞菌的抗菌活性,结果表明金属配合物比席夫碱配体具有更好的抗菌效果。发现金属配合物1 , 2 , 3的催化能力为3 。 > 1 > 2。
更新日期:2021-06-03
中文翻译:

呋喃核席夫碱 Cu (II)、Ni (II) 和 Co (III) 复合物的 DNA 结合和生物活性研究:合成、表征和光谱特性
新颖二元金属络合物,1 [铜(FMIMBMOP)2 ],2 [镍(FMIMBMOP)2 ]和3 [CO(FMIMBMOP)3 ],其中FMIMBMOP(2 - ((E) - ((˚F URAN -2-基)米乙基我氨基)米乙基)-4- b罗莫-6-米ETH ö XY phenol),使用不同的分析技术合成和表征。光谱研究结果表明,Cu (II) 和 Ni (II) 配合物为方形平面几何形状,而 Co (III) 配合物为八面体几何形状。复合物降解的热力学和动力学参数通过 Coats-Redfern 方法确定,用于从热重分析 (TGA) 获得的热重数据。使用最高占据分子轨道 (HOMO)-最低未占据分子轨道 (LUMO) 能量从量子化学参数计算了配合物的稳定性。使用紫外-可见(UV-Vis)光谱技术研究了复合物的DNA结合;ķ b值是2.59±0.01×10 4,7.43±0.03×10 3,和6.73±0.02×10 4 中号-1为1,2,和3,分别。Stern–Volmer 猝灭常数 ( K sv ) 值范围从 2.32 ± 0.03 × 10 3到 1.66 ± 0.02 × 10 4 M -1计算来自荧光研究的复合物。研究了超螺旋 pBR322 DNA 的氧化和光解裂解,发现复合物有效地裂解了该 DNA。新型金属配合物对 DPPH 自由基显示出显着的抗氧化活性。考察了席夫碱及其金属配合物对苏云金芽孢杆菌、肺炎链球菌、大肠杆菌和恶臭假单胞菌的抗菌活性,结果表明金属配合物比席夫碱配体具有更好的抗菌效果。发现金属配合物1 , 2 , 3的催化能力为3 。 > 1 > 2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号