Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH Changes in the Micelle–Water Interface of Surface-Active Ionic Liquids Dictate the Stability of Encapsulated Curcumin: An Insight Through a Unique Interfacial Reaction between Arenediazonium Ions and t-Butyl Hydroquinone
ACS Omega ( IF 3.7 ) Pub Date : 2021-06-03 , DOI: 10.1021/acsomega.1c01119 Saima Afzal 1 , Mohd Sajid Lone 1 , Nighat Nazir 2 , Aijaz Ahmad Dar 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-06-03 , DOI: 10.1021/acsomega.1c01119 Saima Afzal 1 , Mohd Sajid Lone 1 , Nighat Nazir 2 , Aijaz Ahmad Dar 1
Affiliation
|
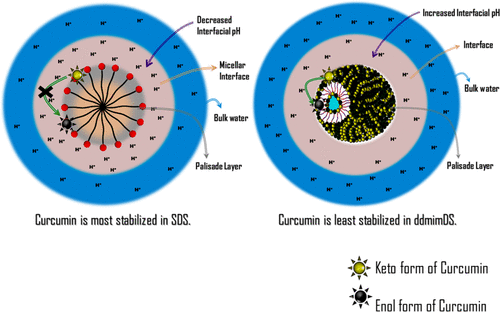
The chemical kinetic (CK) method, which involves the reduction of 4-hexadecylbenzenediazonium ions (16-ArN2+) by antioxidants (in the present case, TBHQ) occurring exclusively at the interface of the association colloids, was employed to establish the changes in the chemical reactivity of anionic surface-active ionic liquids (SAILs) as a function of the concentration and the composition in their mixed states. We used sodium dodecyl sulfate and different SAILs based on the dodecylsulfate surfactant containing 1-alkyl-3-methylimidazolium cations as counterions having a varying alkyl chain length of 4 (bmim), 8 (omim), and 12 (ddmim) carbon atoms. The structural transitions of aggregates of the SAILs from the micellar to vesicular form were observed as a function of concentration in single surfactant systems and as a function of composition in mixed surfactant systems. Results of the reduction kinetics of 16-ArN2+ at the interface of such aggregates, which depends on the acid/base equilibria at the interface, gave an insight into the changes in the interfacial H+ ions with the change in the hydrophobicity of the counterions of SAILs and the morphological changes from micelles to vesicles as a function of concentration or composition. These changes in the interfacial pH correlate very well with the stability of curcumin within these self-assemblies, which exclusively depends on the pH of the medium and highlights the importance of the results obtained from the CK method in selecting the appropriate medium/conditions for the stabilization of the bioactive molecules.
中文翻译:

表面活性离子液体胶束-水界面的 pH 值变化决定了封装姜黄素的稳定性:通过芳烃重氮离子和叔丁基对苯二酚之间独特的界面反应洞察
化学动力学 (CK) 方法,涉及还原 4-十六烷基苯重氮离子 (16-ArN 2 +) 通过仅出现在缔合胶体界面的抗氧化剂(在本例中为 TBHQ),用于确定阴离子表面活性离子液体 (SAIL) 的化学反应性随浓度和组成的变化在他们的混合状态。我们使用十二烷基硫酸钠和基于十二烷基硫酸盐表面活性剂的不同 SAIL,其中含有 1-烷基-3-甲基咪唑鎓阳离子作为抗衡离子,具有不同的烷基链长度,分别为 4 (bmim)、8 (omim) 和 12 (ddmim) 碳原子。观察到 SAIL 的聚集体从胶束到囊泡形式的结构转变作为单一表面活性剂系统中浓度的函数和混合表面活性剂系统中组成的函数。16-ArN 2 +的还原动力学结果在这种聚集体的界面处,取决于界面处的酸/碱平衡,深入了解界面 H +离子随着 SAIL 反离子疏水性的变化以及从胶束到囊泡的形态变化作为浓度或组成的函数。界面 pH 值的这些变化与这些自组装体中姜黄素的稳定性非常相关,这完全取决于培养基的 pH 值,并突出了从 CK 方法获得的结果在选择合适的培养基/条件时的重要性。生物活性分子的稳定性。
更新日期:2021-06-15
中文翻译:

表面活性离子液体胶束-水界面的 pH 值变化决定了封装姜黄素的稳定性:通过芳烃重氮离子和叔丁基对苯二酚之间独特的界面反应洞察
化学动力学 (CK) 方法,涉及还原 4-十六烷基苯重氮离子 (16-ArN 2 +) 通过仅出现在缔合胶体界面的抗氧化剂(在本例中为 TBHQ),用于确定阴离子表面活性离子液体 (SAIL) 的化学反应性随浓度和组成的变化在他们的混合状态。我们使用十二烷基硫酸钠和基于十二烷基硫酸盐表面活性剂的不同 SAIL,其中含有 1-烷基-3-甲基咪唑鎓阳离子作为抗衡离子,具有不同的烷基链长度,分别为 4 (bmim)、8 (omim) 和 12 (ddmim) 碳原子。观察到 SAIL 的聚集体从胶束到囊泡形式的结构转变作为单一表面活性剂系统中浓度的函数和混合表面活性剂系统中组成的函数。16-ArN 2 +的还原动力学结果在这种聚集体的界面处,取决于界面处的酸/碱平衡,深入了解界面 H +离子随着 SAIL 反离子疏水性的变化以及从胶束到囊泡的形态变化作为浓度或组成的函数。界面 pH 值的这些变化与这些自组装体中姜黄素的稳定性非常相关,这完全取决于培养基的 pH 值,并突出了从 CK 方法获得的结果在选择合适的培养基/条件时的重要性。生物活性分子的稳定性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号