当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of Novel Pyrimido[4,5-b]indole Derivatives Against Gram-Negative Multidrug-Resistant Pathogens
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-06-03 , DOI: 10.1021/acs.jmedchem.1c00621 Qidi Kong 1, 2 , Wei Pan 1 , Heng Xu 3 , Yaru Xue 1 , Bin Guo 1 , Xin Meng 1 , Cheng Luo 1, 3 , Ting Wang 4 , Shuhua Zhang 4 , Yushe Yang 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-06-03 , DOI: 10.1021/acs.jmedchem.1c00621 Qidi Kong 1, 2 , Wei Pan 1 , Heng Xu 3 , Yaru Xue 1 , Bin Guo 1 , Xin Meng 1 , Cheng Luo 1, 3 , Ting Wang 4 , Shuhua Zhang 4 , Yushe Yang 1, 2
Affiliation
|
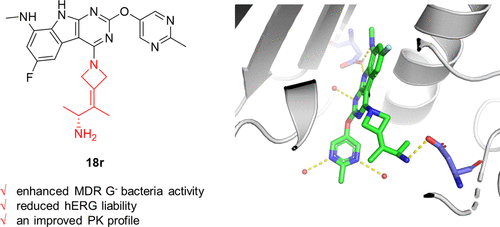
Due to the poor permeability across Gram-negative bacterial membranes and the troublesome bacterial efflux mechanism, only a few GyrB/ParE inhibitors with potent activity against Gram-negative pathogens have been reported. Among them, pyrimido[4,5-b]indole derivatives represented by GP-1 demonstrated excellent broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria but were limited by hERG inhibition and poor pharmacokinetics profile. To improve their drug-like properties, we designed a series of novel pyrimido[4,5-b]indole derivatives based on the tricyclic scaffold of GP-1 and the C-7 moiety of acorafloxacin. These efforts have culminated in the discovery of a promising compound 18r with reduced hERG liability and an improved PK profile. Compound 18r exhibited superior broad-spectrum in vitro antibacterial activity compared to GP-1, including a variety of clinical multidrug G– pathogens, especially Acinetobacter baumannii, and the in vivo efficacy was also demonstrated in a neutropenic mouse thigh model of infection with multidrug-resistant A. baumannii.
中文翻译:

新型嘧啶并[4,5-b]吲哚衍生物抗革兰氏阴性多重耐药病原体的设计、合成和生物学评价
由于革兰氏阴性细菌膜的渗透性差和细菌外排机制麻烦,因此仅报道了少数对革兰氏阴性病原体具有有效活性的 GyrB/ParE 抑制剂。其中,以GP-1为代表的嘧啶并[4,5- b ]吲哚衍生物对革兰氏阳性菌和革兰氏阴性菌均表现出优异的广谱抗菌活性,但受到hERG抑制和较差的药代动力学特征的限制。为了改善它们的类药物特性,我们设计了一系列基于 GP-1 的三环支架和阿卡沙星的 C-7 部分的新型嘧啶并 [4,5- b ] 吲哚衍生物。这些努力最终导致了有希望的化合物18r的发现减少 hERG 责任和改善 PK 概况。化合物18R表现出优异的广谱体外相比GP-1的抗菌活性,包括各种临床耐药的G -病原体,尤其是鲍曼不动杆菌,并且在体内的功效还证实在中性粒细胞减少一个小鼠感染的多药大腿模型耐药鲍曼不动杆菌。
更新日期:2021-06-24
中文翻译:

新型嘧啶并[4,5-b]吲哚衍生物抗革兰氏阴性多重耐药病原体的设计、合成和生物学评价
由于革兰氏阴性细菌膜的渗透性差和细菌外排机制麻烦,因此仅报道了少数对革兰氏阴性病原体具有有效活性的 GyrB/ParE 抑制剂。其中,以GP-1为代表的嘧啶并[4,5- b ]吲哚衍生物对革兰氏阳性菌和革兰氏阴性菌均表现出优异的广谱抗菌活性,但受到hERG抑制和较差的药代动力学特征的限制。为了改善它们的类药物特性,我们设计了一系列基于 GP-1 的三环支架和阿卡沙星的 C-7 部分的新型嘧啶并 [4,5- b ] 吲哚衍生物。这些努力最终导致了有希望的化合物18r的发现减少 hERG 责任和改善 PK 概况。化合物18R表现出优异的广谱体外相比GP-1的抗菌活性,包括各种临床耐药的G -病原体,尤其是鲍曼不动杆菌,并且在体内的功效还证实在中性粒细胞减少一个小鼠感染的多药大腿模型耐药鲍曼不动杆菌。































 京公网安备 11010802027423号
京公网安备 11010802027423号