当前位置:
X-MOL 学术
›
ACS ES&T Water
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanism Investigation of Selective Arsenite Oxidation by Reactive Iodine Species in Hydrogen Peroxide and Iodide (H2O2/I–) System
ACS ES&T Water ( IF 4.8 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsestwater.1c00062 Zhihao Chen 1 , Jingyi Li 1, 2 , Kok Yuen Koh 1, 2 , Zhongrong Du 1, 2 , Choon Nam Ong 1, 3 , J. Paul Chen 1, 2
ACS ES&T Water ( IF 4.8 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsestwater.1c00062 Zhihao Chen 1 , Jingyi Li 1, 2 , Kok Yuen Koh 1, 2 , Zhongrong Du 1, 2 , Choon Nam Ong 1, 3 , J. Paul Chen 1, 2
Affiliation
|
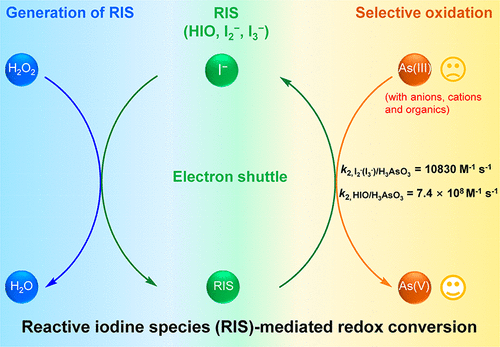
Pretreatment of arsenite (As(III)) to arsenate (As(V)) is crucial in remediation of As(III)-contaminated waterbodies. Hydroxyl or sulfate radicals-based advanced oxidation processes are effective; however, the efficiency becomes low as radicals can be quenched by coexisting matters besides As(III). Both I– and As(III) coexist in several types of contaminated water such as shale gas flowback water. Herein, we proposed using H2O2 to react with I– so as to generate reactive iodine species (RIS) for effective oxidation of As(III). The presence of commonly existing anions, methanol, tert-butanol, 2-propanol, formic acid, humic acid, phenol, and benzoquinone did not affect the oxidation performance. Chromate, Cu2+ or divalent/trivalent irons would enhance the oxidation. Only hydroquinone (>0.3 mM) had an inhibition effect on the oxidation. Unlike peroxymonosulfate and peroxydisulfate that are reactive to Cl– or Br–, H2O2 only showed a solely selective reactivity for the I– to form the RIS. Our experimental and kinetic simulation results confirmed the most significant reaction pathway and suggested that I2 and I3– were the main responsible intermediates. Our study demonstrated that the RIS possessed a higher selectivity to As(III) compared with other coexisting matters. Therefore, the application of RIS is very promising in the redox conversion mediation.
中文翻译:

过氧化氢和碘化物(H 2 O 2 /I –)体系中活性碘选择性氧化亚砷酸盐的动力学和机理研究
将亚砷酸盐 (As(III)) 预处理为砷酸盐 (As(V)) 对于修复受 As(III) 污染的水体至关重要。基于羟基或硫酸根的高级氧化工艺是有效的;然而,效率变低,因为自由基可以被除 As(III) 以外的共存物质淬灭。I -和 As(III) 共存于多种类型的污染水中,例如页岩气返排水。在此,我们提出了使用H 2 ö 2与我反应-从而产生反应性的碘物质(RIS)对As(III)的有效氧化。常见阴离子、甲醇、叔丁醇、2-丙醇、甲酸、腐植酸、苯酚和苯醌的存在不影响氧化性能。铬酸盐,Cu 2+或二价/三价铁会增强氧化。只有对苯二酚 (>0.3 mM) 对氧化有抑制作用。与对 Cl -或 Br -具有反应性的过硫酸盐和过硫酸盐不同,H 2 O 2仅对 I -显示出选择性反应性,以形成 RIS。我们的实验和动力学模拟结果证实了最重要的反应途径,并表明 I 2和 I 3 –是主要负责的中间体。我们的研究表明,与其他共存物质相比,RIS 对 As(III) 具有更高的选择性。因此,RIS在氧化还原转化介导方面的应用前景广阔。
更新日期:2021-06-11
中文翻译:

过氧化氢和碘化物(H 2 O 2 /I –)体系中活性碘选择性氧化亚砷酸盐的动力学和机理研究
将亚砷酸盐 (As(III)) 预处理为砷酸盐 (As(V)) 对于修复受 As(III) 污染的水体至关重要。基于羟基或硫酸根的高级氧化工艺是有效的;然而,效率变低,因为自由基可以被除 As(III) 以外的共存物质淬灭。I -和 As(III) 共存于多种类型的污染水中,例如页岩气返排水。在此,我们提出了使用H 2 ö 2与我反应-从而产生反应性的碘物质(RIS)对As(III)的有效氧化。常见阴离子、甲醇、叔丁醇、2-丙醇、甲酸、腐植酸、苯酚和苯醌的存在不影响氧化性能。铬酸盐,Cu 2+或二价/三价铁会增强氧化。只有对苯二酚 (>0.3 mM) 对氧化有抑制作用。与对 Cl -或 Br -具有反应性的过硫酸盐和过硫酸盐不同,H 2 O 2仅对 I -显示出选择性反应性,以形成 RIS。我们的实验和动力学模拟结果证实了最重要的反应途径,并表明 I 2和 I 3 –是主要负责的中间体。我们的研究表明,与其他共存物质相比,RIS 对 As(III) 具有更高的选择性。因此,RIS在氧化还原转化介导方面的应用前景广阔。































 京公网安备 11010802027423号
京公网安备 11010802027423号