Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-06-01 , DOI: 10.1016/j.foodhyd.2021.106906 Chengzhi Liu , Nan Lv , Gerui Ren , Ruibo Wu , Binju Wang , Zexing Cao , Hujun Xie
|
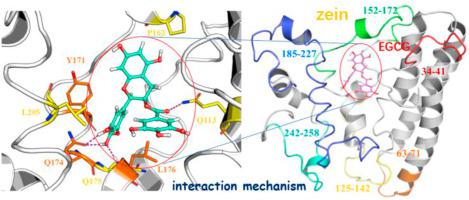
Zein as a kind of carrier material has attracted extensive interest, while the interaction mechanism between zein and nutrients is still elusive. In this work, the binding mechanism of zein with epigallocatechin-3-gallate (EGCG) was investigated via multi-spectroscopy and molecular dynamics (MD) simulation. The quenching of zein by EGCG is mainly static quenching, and the secondary structure of zein is slightly changed after the binding of EGCG to zein. The formation of Zein-EGCG complexes was confirmed by ultraviolet–visible (UV–Vis) spectroscopy, X-ray diffraction (XRD), and scanning electron microscopy (SEM). Molecular dynamics (MD) simulation clarified that the EGCG prefers to bind to the pocket of zein formed by residues Y171, Q174, L176 and L205. Electrostatic and van der Waals interactions played a dominant role for the binding of EGCG to zein, which was consistent with the results by fourier transform infrared spectroscopy (FTIR) and thermodynamic analysis. This study provides new insights into the interaction mechanism between zein and EGCG, which is very important to develop plant protein as tea polyphenol delivery system.
中文翻译:

使用多光谱和分子动力学模拟方法探索玉米醇溶蛋白与 EGCG 之间的相互作用机制
玉米蛋白作为一种载体材料引起了广泛的兴趣,而玉米蛋白与营养物质之间的相互作用机制尚不清楚。在这项工作中,通过多光谱和分子动力学 (MD) 模拟研究了玉米醇溶蛋白与表没食子儿茶素-3-没食子酸酯 (EGCG) 的结合机制。EGCG对玉米蛋白的猝灭主要是静态猝灭,EGCG与玉米蛋白结合后,玉米蛋白的二级结构略有变化。玉米蛋白-EGCG 复合物的形成通过紫外-可见(UV-Vis)光谱、X 射线衍射(XRD)和扫描电子显微镜(SEM)证实。分子动力学 (MD) 模拟阐明 EGCG 更喜欢与由 Y171、Q174、L176 和 L205 残基形成的玉米醇溶蛋白口袋结合。静电和范德华相互作用对 EGCG 与玉米醇溶蛋白的结合起主导作用,这与傅里叶变换红外光谱 (FTIR) 和热力学分析的结果一致。该研究为玉米醇溶蛋白与 EGCG 之间的相互作用机制提供了新的见解,这对于开发植物蛋白作为茶多酚传递系统非常重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号