当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Methane Oxidation Greatly Promoted by Chlorine Intermediates
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-06-01 , DOI: 10.1002/anie.202105523 Qihao Wang 1 , Tengfei Li 1 , Chao Yang 1 , Menghuan Chen 1 , Anxiang Guan 1 , Li Yang 1 , Si Li 1 , Ximeng Lv 1 , Yuhang Wang 2 , Gengfeng Zheng 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-06-01 , DOI: 10.1002/anie.202105523 Qihao Wang 1 , Tengfei Li 1 , Chao Yang 1 , Menghuan Chen 1 , Anxiang Guan 1 , Li Yang 1 , Si Li 1 , Ximeng Lv 1 , Yuhang Wang 2 , Gengfeng Zheng 1
Affiliation
|
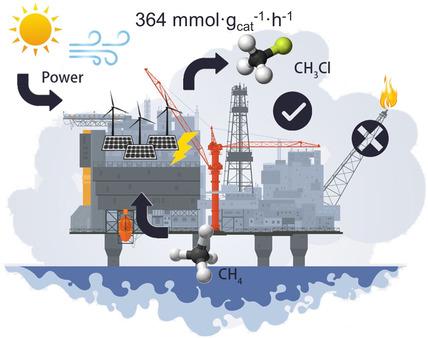
Renewable energy-powered methane (CH4) conversion at ambient conditions is an attractive but highly challenging field. Due to the highly inert character of CH4, the selective cleavage of its first C−H bond without over-oxidation is essential for transforming CH4 into value-added products. In this work, we developed an efficient and selective CH4 conversion approach at room temperature using intermediate chlorine species (*Cl), which were electrochemically generated and stabilized on mixed cobalt–nickel spinels with different Co/Ni ratios. The lower overpotentials for *Cl formation enabled an effective activation and conversion of CH4 to CH3Cl without over-oxidation to CO2, and Ni3+ at the octahedral sites in the mixed cobalt–nickel spinels allowed to stabilize surface-bound *Cl species. The CoNi2Ox electrocatalyst exhibited an outstanding yield of CH3Cl (364 mmol g−1 h−1) and a high CH3Cl/CO2 selectivity of over 400 at room temperature, with demonstrated capability of direct CH4 conversion under seawater working conditions.
中文翻译:

氯中间体极大地促进了电催化甲烷氧化
在环境条件下可再生能源驱动的甲烷 (CH 4 ) 转化是一个有吸引力但极具挑战性的领域。由于 CH 4的高度惰性特性,其第一个 CH 键的选择性裂解而不过度氧化对于将 CH 4转化为增值产品至关重要。在这项工作中,我们开发了一种在室温下使用中间氯物质 (*Cl)的高效且选择性 CH 4转化方法,这些物质是在具有不同 Co/Ni 比率的混合钴镍尖晶石上电化学生成和稳定的。*Cl 形成的较低过电位能够有效地激活 CH 4并将其转化为 CH 3 Cl,而不会过度氧化为 CO2,以及混合钴镍尖晶石中八面体位点的Ni 3+允许稳定表面结合的 *Cl 物种。所述的CoNi 2 ö X电展出CH的杰出收率3氯(364毫摩尔克-1 ħ -1)和高CH 3 CL / CO 2在室温下超过400的选择性,与直接CH的证明能力4下转换海水工作条件。
更新日期:2021-07-27
中文翻译:

氯中间体极大地促进了电催化甲烷氧化
在环境条件下可再生能源驱动的甲烷 (CH 4 ) 转化是一个有吸引力但极具挑战性的领域。由于 CH 4的高度惰性特性,其第一个 CH 键的选择性裂解而不过度氧化对于将 CH 4转化为增值产品至关重要。在这项工作中,我们开发了一种在室温下使用中间氯物质 (*Cl)的高效且选择性 CH 4转化方法,这些物质是在具有不同 Co/Ni 比率的混合钴镍尖晶石上电化学生成和稳定的。*Cl 形成的较低过电位能够有效地激活 CH 4并将其转化为 CH 3 Cl,而不会过度氧化为 CO2,以及混合钴镍尖晶石中八面体位点的Ni 3+允许稳定表面结合的 *Cl 物种。所述的CoNi 2 ö X电展出CH的杰出收率3氯(364毫摩尔克-1 ħ -1)和高CH 3 CL / CO 2在室温下超过400的选择性,与直接CH的证明能力4下转换海水工作条件。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号