当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Ternary Phase Equilibrium of 3,5-Dibromo-4-hydroxybenzaldehyde + 3-Bromo-4-hydroxybenzaldehyde + N,N-Dimethylformamide/1,4-dioxane/dimethylsulfoxide: Determination and Model Correlation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-05-30 , DOI: 10.1021/acs.jced.1c00222 Huanxin Li 1 , Yingnan Xie 1 , Yan Xue 1 , Peizhi Zhu 2 , Hongkun Zhao 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-05-30 , DOI: 10.1021/acs.jced.1c00222 Huanxin Li 1 , Yingnan Xie 1 , Yan Xue 1 , Peizhi Zhu 2 , Hongkun Zhao 2
Affiliation
|
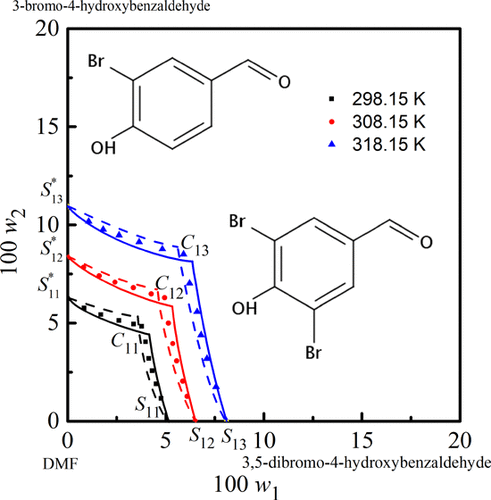
The solid–liquid ternary phase equilibrium for 3,5-dibromo-4-hydroxybenzaldehyde + 3-bromo-4-hydroxybenzaldehyde + N,N-dimethylformamide/1,4-dioxane/dimethylsulfoxide systems was experimentally obtained through the shake-flask method at three temperatures of 298.15, 308.15, and 318.15 K under a normal pressure of 101.2 kPa. Nine isothermal phase diagrams of the three systems were constructed by means of the mutual solubility data acquired. The equilibrated solids with different solvent systems were identified by Schreinemakers’ wet residue method. Two neat solids corresponding to pure 3-bromo-4-hydroxybenzaldehyde and pure 3,5-dibromo-4-hydroxybenzaldehyde appeared in each equilibrium system at a specific temperature. The saturated regions of pure 3-bromo-4-hydroxybenzaldehyde and 3,5-dibromo-4-hydroxybenzaldehyde positively increased as the temperature decreased. The saturated region of 3,5-dibromo-4-hydroxybenzaldehyde was bigger than that of 3-bromo-4-hydroxybenzaldehyde at the same conditions. Two activity coefficient models, NRTL and Wilson models, were utilized to mathematically describe the solid–liquid phase equilibrium. The back-calculated solubility data through the NRTL model were in good agreement with the experiment ones. The ternary phase diagrams and mutual solubility data for these systems are crucial in the purification of 3-bromo-4-hydroxybenzaldehyde and 3,5-dibromo-4-hydroxybenzaldehyde mixtures.
中文翻译:

3,5-二溴-4-羟基苯甲醛+3-溴-4-羟基苯甲醛+ N,N-二甲基甲酰胺/1,4-二恶烷/二甲亚砜的固液三元相平衡:测定和模型相关性
3,5-二溴-4-羟基苯甲醛+3-溴-4-羟基苯甲醛+ N,N的固液三元相平衡通过摇瓶法在298.15、308.15和318.15 K三个温度、101.2 kPa常压下实验得到-二甲基甲酰胺/1,4-二恶烷/二甲基亚砜体系。通过获得的互溶数据构建了三个体系的九个等温相图。用 Schreinemakers 湿法残留物法鉴定具有不同溶剂系统的平衡固体。对应于纯 3-溴-4-羟基苯甲醛和纯 3,5-二溴-4-羟基苯甲醛的两种纯固体在特定温度下出现在每个平衡系统中。纯3-溴-4-羟基苯甲醛和3,5-二溴-4-羟基苯甲醛的饱和区域随着温度的降低而正增加。3的饱和区域,在相同条件下,5-二溴-4-羟基苯甲醛大于3-溴-4-羟基苯甲醛。两个活度系数模型,NRTL 和威尔逊模型,被用来数学描述固液相平衡。通过 NRTL 模型反算的溶解度数据与实验数据吻合良好。这些系统的三元相图和互溶数据对于 3-溴-4-羟基苯甲醛和 3,5-二溴-4-羟基苯甲醛混合物的纯化至关重要。
更新日期:2021-06-10
中文翻译:

3,5-二溴-4-羟基苯甲醛+3-溴-4-羟基苯甲醛+ N,N-二甲基甲酰胺/1,4-二恶烷/二甲亚砜的固液三元相平衡:测定和模型相关性
3,5-二溴-4-羟基苯甲醛+3-溴-4-羟基苯甲醛+ N,N的固液三元相平衡通过摇瓶法在298.15、308.15和318.15 K三个温度、101.2 kPa常压下实验得到-二甲基甲酰胺/1,4-二恶烷/二甲基亚砜体系。通过获得的互溶数据构建了三个体系的九个等温相图。用 Schreinemakers 湿法残留物法鉴定具有不同溶剂系统的平衡固体。对应于纯 3-溴-4-羟基苯甲醛和纯 3,5-二溴-4-羟基苯甲醛的两种纯固体在特定温度下出现在每个平衡系统中。纯3-溴-4-羟基苯甲醛和3,5-二溴-4-羟基苯甲醛的饱和区域随着温度的降低而正增加。3的饱和区域,在相同条件下,5-二溴-4-羟基苯甲醛大于3-溴-4-羟基苯甲醛。两个活度系数模型,NRTL 和威尔逊模型,被用来数学描述固液相平衡。通过 NRTL 模型反算的溶解度数据与实验数据吻合良好。这些系统的三元相图和互溶数据对于 3-溴-4-羟基苯甲醛和 3,5-二溴-4-羟基苯甲醛混合物的纯化至关重要。










































 京公网安备 11010802027423号
京公网安备 11010802027423号