当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting Anion–π Interactions for Efficient and Selective Catalysis with Chiral Molecular Cages
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-29 , DOI: 10.1002/anie.202106509 Na Luo 1, 2 , Yu-Fei Ao 1, 2 , De-Xian Wang 1, 2 , Qi-Qiang Wang 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-29 , DOI: 10.1002/anie.202106509 Na Luo 1, 2 , Yu-Fei Ao 1, 2 , De-Xian Wang 1, 2 , Qi-Qiang Wang 1, 2
Affiliation
|
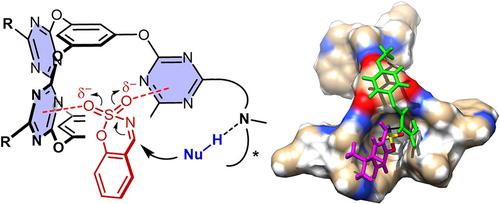
Exploiting anion–π interactions in catalyst design is a fascinating direction to develop new and fundamental catalysis. For the appealing yet flexible π-face activation, can two or more π-acidic surfaces be manipulated for cooperative activation to achieve efficient transformation and particularly selectivity control is highly desirable. Here, we demonstrate a supramolecular π-catalysis strategy by establishing cooperative π-face activation in a confined electron-deficient cage cavity. The catalysts have a triazine based prism-like cage core and pendant chiral base sites. Only 2 mol % of cage catalyst efficiently catalyzed the decarboxylate Mannich reactions of sulfamate-headed cyclic aldimines and a series of malonic acid half thioesters in nearly quantitative yields and up to 97 % ee, enabling an unprecedent organocatalytic approach. The supramolecular π-cavity is essential in harnessing cooperative anion–π interactions for the efficient activation and excellent selectivity control.
中文翻译:

手性分子笼利用阴离子-π相互作用进行高效和选择性催化
在催化剂设计中利用阴离子-π 相互作用是开发新的基础催化的一个引人入胜的方向。对于吸引人但灵活的 π 面激活,可以操纵两个或多个 π 酸性表面进行协同激活以实现有效的转化,特别是选择性控制是非常需要的。在这里,我们通过在受限的缺电子笼腔中建立协作 π 面激活来展示超分子 π 催化策略。该催化剂具有基于三嗪的棱柱状笼核和悬垂的手性碱基位点。仅 2 mol % 的笼状催化剂有效催化氨基磺酸酯头环醛亚胺和一系列丙二酸半硫酯的脱羧曼尼希反应,收率几乎是定量的,ee可达 97 %,实现了前所未有的有机催化方法。超分子 π 腔对于利用协同阴离子-π 相互作用进行有效活化和出色的选择性控制至关重要。
更新日期:2021-05-29
中文翻译:

手性分子笼利用阴离子-π相互作用进行高效和选择性催化
在催化剂设计中利用阴离子-π 相互作用是开发新的基础催化的一个引人入胜的方向。对于吸引人但灵活的 π 面激活,可以操纵两个或多个 π 酸性表面进行协同激活以实现有效的转化,特别是选择性控制是非常需要的。在这里,我们通过在受限的缺电子笼腔中建立协作 π 面激活来展示超分子 π 催化策略。该催化剂具有基于三嗪的棱柱状笼核和悬垂的手性碱基位点。仅 2 mol % 的笼状催化剂有效催化氨基磺酸酯头环醛亚胺和一系列丙二酸半硫酯的脱羧曼尼希反应,收率几乎是定量的,ee可达 97 %,实现了前所未有的有机催化方法。超分子 π 腔对于利用协同阴离子-π 相互作用进行有效活化和出色的选择性控制至关重要。






































 京公网安备 11010802027423号
京公网安备 11010802027423号