Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-05-29 , DOI: 10.1016/j.jcat.2021.05.022
Yian Wang , Xueping Qin , Minhua Shao
|
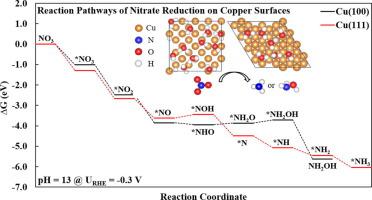
As a beneficial technology for nitrate removal and ammonia synthesis, electrocatalytic nitrate reduction reaction (NO3RR) has received much attention in experimental and theoretical studies. Previous work suggests that Cu is one of the most active electrocatalysts for NO3RR. However, the fundamental reaction mechanism, governing the activity and selectivity of Cu towards NO3RR with various facets and pH, remains elusive. Herein, a hybrid model is developed by combining the explicit water layer and the implicit solvation scheme to simulate the water/Cu interface based on theoretical density functional theory simulations. The hybrid model is used to elucidate the effects of crystal facets and pH on NO3RR mechanism over Cu. Our simulation results illustrate that NO3RR in acidic media proceeds via the formation of *NOH to produce NH3 on both (1 0 0) and (1 1 1) facets. While in alkaline media, Cu(1 0 0) prefers the formation of NH2OH via *NHO at low overpotentials, and Cu(1 1 1) kinetically favors the formation of NH3. In general, the (1 0 0) facet is more active towards NO3RR than the (1 1 1) facet, especially at higher pH, due to lower thermodynamic and kinetic barriers. Our results provide deeper mechanistic insights into the effects of both crystal facets and pH on the electrocatalytic activity and product selectivity of Cu towards NO3RR. The hybrid model developed here can be applied to other electrochemical reactions at water/metal interfaces.
中文翻译:

铜表面硝酸盐还原反应的第一性原理机理研究:晶面和pH值的影响
作为去除硝酸盐和合成氨的有益技术,电催化硝酸盐还原反应(NO 3 RR)在实验和理论研究中备受关注。先前的工作表明,Cu 是 NO 3 RR最活跃的电催化剂之一。然而,控制 Cu 对具有不同方面和 pH 值的NO 3 RR的活性和选择性的基本反应机制仍然难以捉摸。在此,基于理论密度泛函理论模拟,通过结合显式水层和隐式溶剂化方案来模拟水/铜界面,开发了混合模型。混合模型用于阐明晶面和 pH 值对 NO 3 的影响Cu上的RR机制。我们的模拟结果表明,酸性介质中的 NO 3 RR 通过形成 *NOH 进行,从而在 (1 0 0) 和 (1 1 1) 面生成 NH 3。而在碱性介质中,Cu(1 0 0) 倾向于在低过电位下通过 *NHO形成 NH 2 OH,而 Cu(1 1 1) 在动力学上有利于 NH 3的形成。一般来说,(1 0 0)面对NO 3 RR 比 (1 1 1) 由于较低的热力学和动力学障碍,面,尤其是在较高的 pH 值下。我们的结果为了解晶面和 pH 值对 Cu 对 NO 3 RR的电催化活性和产物选择性的影响提供了更深入的机理见解。此处开发的混合模型可应用于水/金属界面处的其他电化学反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号