Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-05-27 , DOI: 10.1016/j.bioorg.2021.105033 Botros Y Beshay 1 , Amira A Abdellatef 2 , Yasser M Loksha 3 , Salwa M Fahmy 4 , Nargues S Habib 4 , Alaa El-Din A Bekhit 5 , Paris E Georghiou 6 , Yoshihiro Hayakawa 2 , Adnan A Bekhit 7
|
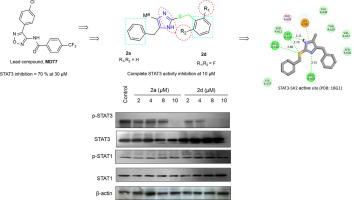
STAT3 signaling is known to be associated with tumorigenesis and further cancer cell-intrinsic activation of STAT3 leads to altered regulation of several oncogenic processes. Given the importance of STAT3 in cancer development and progression particularly breast cancer, it is crucial to discover new chemical entities of STAT3 inhibitor to develop anti-breast cancer drug candidates. Herein, 4-benzyl-2-benzylthio-5-methyl-1H-imidazole (2a) and 4-benzyl-5-methyl-2-[(2,6-difluorobenzyl)thio]-1H-imidazole (2d) from a group of thirty imidazole-bearing compounds showed greater STAT3 inhibition than their lead compounds VS1 and the oxadiazole derivative MD77. Within all tested compounds, ten derivatives effectively inhibited the growth of the two tested breast cancer cells with IC50 values ranging from 6.66 to 26.02 µM. In addition, the most potent derivatives 2a and 2d inhibited the oncogenic function of STAT3 as seen in the inhibition of colony formation and IL-6 production of breast cancer cell lines. Modeling studies provided evidence for the possible interactions of the synthesized compounds with the key residues of the STAT3-SH2 domain. Collectively, our present study suggests 2-substituted-4-benzyl-5-methylimidazoles are a new class of anti-cancer drug candidates to inhibit oncogenic STAT3 function.
中文翻译:

设计和合成 2-Substituted-4-benzyl-5-methylimidazoles 作为抑制致癌 STAT3 功能的新型潜在抗乳腺癌药物
已知 STAT3 信号转导与肿瘤发生相关,并且进一步的癌细胞内在激活 STAT3 会导致对几种致癌过程的调节发生改变。鉴于 STAT3 在癌症发展和进展中的重要性,尤其是乳腺癌,发现 STAT3 抑制剂的新化学实体以开发抗乳腺癌候选药物至关重要。在此,4-benzyl-2-benzylthio-5-methyl-1 H - imidazole ( 2a ) 和 4-benzyl-5-methyl-2-[(2,6-difluorobenzyl)thio]-1 H- imidazole ( 2d)) 来自一组 30 个含咪唑的化合物显示出比它们的先导化合物 VS1 和恶二唑衍生物 MD77 更大的 STAT3 抑制。在所有测试化合物中,十种衍生物有效抑制了两种测试乳腺癌细胞的生长,IC 50值范围为 6.66 至 26.02 µM。此外,最有效的衍生物2a和2d抑制 STAT3 的致癌功能,如抑制乳腺癌细胞系的集落形成和 IL-6 产生所见。建模研究为合成化合物与 STAT3-SH2 结构域的关键残基可能存在相互作用提供了证据。总的来说,我们目前的研究表明 2-取代-4-苄基-5-甲基咪唑是一类新的抗癌候选药物,可抑制致癌 STAT3 功能。







































 京公网安备 11010802027423号
京公网安备 11010802027423号