Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2021-07-31 , DOI: 10.2174/1573412916999200616124632 Yu Li 1 , Xiangwen Kong 2 , Liya Hong 1 , Chen Yue 1 , Xinyue Wang 2 , Peixi Zhu 3
|
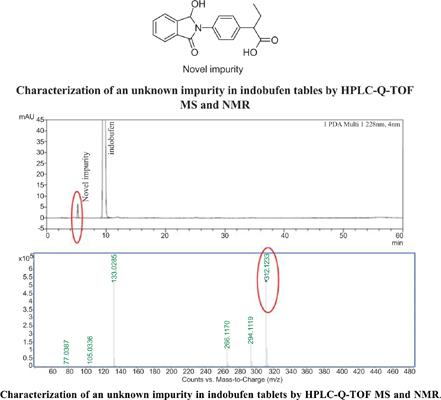
Background: Indobufen is a drug that hinders the aggregation of platelets by reversibly repressing the cyclooxygenase enzyme, further bringing about diminished thromboxane production. During quality control of indobufen tablets, an unknown impurity was detected.
Objective: To characterize an unknown impurity in indobufen tablets.
Methods: A new method compatible with mass spectrometry detection was set up. A C18 column at 35 °C with a mobile phase consisting of aqueous buffer (including ammonium formate) and methanol (35: 65, v/v) was used at a flow rate of 1.0 mL/min at 228 nm. High-performance liquid chromatography quadrupole time-of-flight mass spectrometry mass spectrometry (HPLC-Q-TOF MS) was used to identify the impurity with the electrospray ionization (ESI) source in the positive ionization mode.
Results: The results of HPLC-Q-TOF MS analysis indicated that the protonated molecule ions [M + H]+ of the unknown impurity was at m/z 312. Preparative LC method was put into practice with a Prep- C18 column with a mobile phase consisting of water and methanol (20: 80, v/v) at a flow rate of 20.0 mL/min at 228 nm. The assignment of the 1D and 2D NMR signals was performed for the unknown impurity. In addition, possible formation of the novel impurity was also studied.
Conclusion: An unknown impurity in indobufen tablets was characterized. The impurity was assigned as 2-(4-(1-hydroxy-3-oxoisoindolin-2-yl) phenyl) butanoic acid.
中文翻译:

HPLC-Q-TOF MS和NMR表征吲哚布芬片中的未知杂质
背景:吲哚布芬是通过可逆地抑制环氧合酶来阻止血小板凝集的药物,从而进一步减少了血栓烷的产生。在吲哚布芬片剂的质量控制过程中,检测到未知杂质。
目的:鉴定吲哚布芬片剂中的未知杂质。
方法:建立了一种与质谱检测兼容的新方法。在35°C下使用C18色谱柱,其流动相由水性缓冲液(包括甲酸铵)和甲醇(35:65,v / v)组成,流速为228 nm,流速为1.0 mL / min。高效液相色谱四极杆飞行时间质谱(HPLC-Q-TOF MS)用于以正电离模式通过电喷雾电离(ESI)源鉴定杂质。
结果:HPLC-Q-TOF MS分析的结果表明,未知杂质的质子化分子离子[M + H] +处于m / z 312。流动相由水和甲醇(20:80,v / v)组成,流速为20.0 mL / min,在228 nm处。1D和2D NMR信号的分配是针对未知杂质进行的。此外,还研究了可能形成的新型杂质。
结论:吲哚布芬片中存在未知杂质。杂质被指定为2-(4-(1-羟基-3-氧代异吲哚-2-基)苯基)丁酸。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号