当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of 8-Aminoquinoline-Directed Ni-Catalyzed C(sp3)–H Functionalization: Paramagnetic Ni(II) Species and the Deleterious Effect of Carbonate as a Base
Organometallics ( IF 2.5 ) Pub Date : 2021-05-26 , DOI: 10.1021/acs.organomet.1c00265 Junyang Liu 1 , Samuel A. Johnson 1
Organometallics ( IF 2.5 ) Pub Date : 2021-05-26 , DOI: 10.1021/acs.organomet.1c00265 Junyang Liu 1 , Samuel A. Johnson 1
Affiliation
|
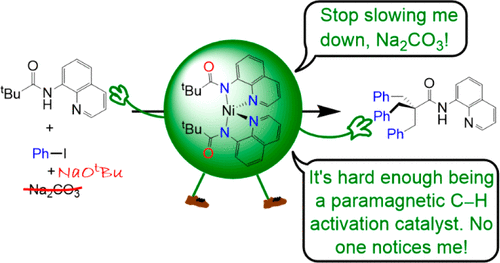
Studies into the mechanism of 8-aminoquinoline-directed nickel-catalyzed C(sp3)–H arylation with iodoarenes were carried out, to determine the catalyst resting state and optimize catalytic performance. Paramagnetic complexes undergo the key C–H activation step. The ubiquitous base Na2CO3 is found to hinder catalysis; replacement of Na2CO3 with NaOtBu gave improved catalytic turnovers under milder conditions. Deprotonation of the 8-aminoquinoline derivative N-(quinolin-8-yl)pivalamide (1a) at the amide nitrogen using NaH, followed by reaction with NiCl2(PPh3)2 allowed for the isolation of complex Ni([AQpiv]-κN,N)2 (3) with chelating N-donors (where [AQpiv] = C9NH6NCOtBu). Complex 3 is a four-coordinate disphenoidal high-spin Ni(II) complex, excluding short anagostic Ni--tBu hydrogen interactions. Complex 3 reacts with the paddle-wheel [Ph3PNi(μ-CO2tBu)2]2 (6·PPh3) or tBuCO2H to give insoluble {[AQpiv]Ni(O2CtBu)}2 (5). Dissolution of 5 in donor solvents L (L= DMSO and DMF) gave a paramagnetic intermediate assigned by NMR as [AQpiv]Ni(O2CtBu)L (5·L) and equilibrium reformation of 3 and 6·L. DFT calculations support this equilibrium in solution. Both 3 and 5 undergo C–H activation at temperatures as low as 80 °C and in the presence of PR3 (PR3 = PPh3, PiBu3) to give Ni(C9NH6NCOCMe2CH2-κN,N,C)PR3 (7·PR3). The C–H functionalization reaction orders with respect to 7·PiBu3, iodoarenes, and phosphines were determined. Hammett analysis using electronically different aryl iodides suggests a concerted oxidative addition mechanism for the C–H functionalization step; DFT calculations were also carried out to support this finding. When Na2CO3 is used as the base, the rate determination step for C–H functionalization appears to be 8-aminoquinoline deprotonation and binding to Ni. The carbonate anion was also observed to provide a deleterious NMR-inactive low-energy off-cycle resting state in catalysis. Replacement of Na2CO3 with NaOtBu improved catalysis at milder conditions and made carboxylic acid and phosphine additives unnecessary. Complex 3 and its functionalized analogues were observed as the catalyst resting state under these conditions.
中文翻译:

8-氨基喹啉导向的镍催化 C(sp3)-H 官能化的机制:顺磁性 Ni(II) 物种和碳酸盐作为碱的有害影响
研究了 8-氨基喹啉导向的镍催化 C(sp 3 )-H 与碘芳烃的芳基化机理,以确定催化剂的静止状态并优化催化性能。顺磁性配合物经历关键的 C-H 激活步骤。发现无处不在的碱 Na 2 CO 3会阻碍催化;在较温和的条件下,用 NaO t Bu替代 Na 2 CO 3提高了催化转化率。使用 NaH 在酰胺氮处对8-氨基喹啉衍生物N- (quinolin-8-yl)pivalamide ( 1a ) 进行去质子化,然后与 NiCl 2 (PPh 3 ) 2 反应允许分离复合物 Ni([AQ piv ]-κ N , N ) 2 ( 3 ) 与螯合 N 供体(其中 [AQ piv ] = C 9 NH 6 NCO t Bu)。配合物3是一个四配位的蝶形高自旋 Ni(II) 配合物,不包括短的 Anagostic Ni-- t Bu 氢相互作用。配合物3与桨轮反应 [Ph 3 PNi(μ-CO 2 t Bu) 2 ] 2 ( 6·PPh 3 ) 或t BuCO 2H 得到不溶性 {[AQ piv ]Ni(O 2 C t Bu)} 2 ( 5 )。5在供体溶剂 L(L = DMSO 和 DMF)中的溶解产生了一种顺磁性中间体,由 NMR 指定为 [AQ piv ]Ni(O 2 C t Bu)L ( 5·L ) 和3和6·L 的平衡重整。DFT 计算支持解决方案中的这种平衡。两个3和5经受C-H活化在温度低至80℃,并在PR的存在3(PR 3 = PPH 3,P我Bu 3 ) 得到Ni(C 9 NH 6 NCOCMe 2 CH 2 -κ N , N , C )PR 3 ( 7·PR 3 )。确定了关于7·P i Bu 3、碘芳烃和膦的 C-H 官能化反应顺序。使用电子上不同的芳基碘化物进行的哈米特分析表明,C-H 功能化步骤存在协同氧化加成机制;还进行了 DFT 计算以支持这一发现。当 Na 2 CO 3用作基础,C-H 功能化的速率确定步骤似乎是 8-氨基喹啉去质子化并与 Ni 结合。还观察到碳酸根阴离子在催化中提供有害的 NMR 非活性低能量非循环静止状态。用 NaO t Bu代替 Na 2 CO 3改善了在温和条件下的催化作用,并且不需要羧酸和膦添加剂。在这些条件下观察到复合物3及其功能化类似物作为催化剂的静止状态。
更新日期:2021-05-26
中文翻译:

8-氨基喹啉导向的镍催化 C(sp3)-H 官能化的机制:顺磁性 Ni(II) 物种和碳酸盐作为碱的有害影响
研究了 8-氨基喹啉导向的镍催化 C(sp 3 )-H 与碘芳烃的芳基化机理,以确定催化剂的静止状态并优化催化性能。顺磁性配合物经历关键的 C-H 激活步骤。发现无处不在的碱 Na 2 CO 3会阻碍催化;在较温和的条件下,用 NaO t Bu替代 Na 2 CO 3提高了催化转化率。使用 NaH 在酰胺氮处对8-氨基喹啉衍生物N- (quinolin-8-yl)pivalamide ( 1a ) 进行去质子化,然后与 NiCl 2 (PPh 3 ) 2 反应允许分离复合物 Ni([AQ piv ]-κ N , N ) 2 ( 3 ) 与螯合 N 供体(其中 [AQ piv ] = C 9 NH 6 NCO t Bu)。配合物3是一个四配位的蝶形高自旋 Ni(II) 配合物,不包括短的 Anagostic Ni-- t Bu 氢相互作用。配合物3与桨轮反应 [Ph 3 PNi(μ-CO 2 t Bu) 2 ] 2 ( 6·PPh 3 ) 或t BuCO 2H 得到不溶性 {[AQ piv ]Ni(O 2 C t Bu)} 2 ( 5 )。5在供体溶剂 L(L = DMSO 和 DMF)中的溶解产生了一种顺磁性中间体,由 NMR 指定为 [AQ piv ]Ni(O 2 C t Bu)L ( 5·L ) 和3和6·L 的平衡重整。DFT 计算支持解决方案中的这种平衡。两个3和5经受C-H活化在温度低至80℃,并在PR的存在3(PR 3 = PPH 3,P我Bu 3 ) 得到Ni(C 9 NH 6 NCOCMe 2 CH 2 -κ N , N , C )PR 3 ( 7·PR 3 )。确定了关于7·P i Bu 3、碘芳烃和膦的 C-H 官能化反应顺序。使用电子上不同的芳基碘化物进行的哈米特分析表明,C-H 功能化步骤存在协同氧化加成机制;还进行了 DFT 计算以支持这一发现。当 Na 2 CO 3用作基础,C-H 功能化的速率确定步骤似乎是 8-氨基喹啉去质子化并与 Ni 结合。还观察到碳酸根阴离子在催化中提供有害的 NMR 非活性低能量非循环静止状态。用 NaO t Bu代替 Na 2 CO 3改善了在温和条件下的催化作用,并且不需要羧酸和膦添加剂。在这些条件下观察到复合物3及其功能化类似物作为催化剂的静止状态。






























 京公网安备 11010802027423号
京公网安备 11010802027423号