当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Aza-Diels–Alder Reaction of Ketimines and Unactivated Dienes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-25 , DOI: 10.1002/anie.202104788 Qun Zhao 1 , Yao Li 1 , Qing-Xia Zhang 1 , Jin-Pei Cheng 1, 2 , Xin Li 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-25 , DOI: 10.1002/anie.202104788 Qun Zhao 1 , Yao Li 1 , Qing-Xia Zhang 1 , Jin-Pei Cheng 1, 2 , Xin Li 1
Affiliation
|
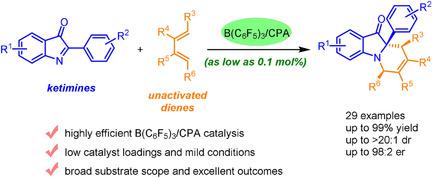
The enantioselective aza-Diels–Alder reaction is efficient for constructing chiral tetrahydropyridines, but the catalytic asymmetric aza-Diels–Alder reaction of ketimines with unactivated dienes is still a challenging topic. Herein, guided by computational screening, a highly enantioselective aza-Diels–Alder reaction of 2-aryl-3H-indol-3-ones with unactivated dienes was realized by using a B(C6F5)3/chiral phosphoric acid catalyst system under mild conditions. The reaction has a broad scope with respect to both aza-Diels–Alder reaction partners and hence offers rapid access to an array of tetrahydropyridine derivatives with pretty outcomes (up to 99 % yield, >20:1 dr and 98:2 er). The reaction is very efficient: lowering catalyst loadings for the model reaction to 0.1 mol %, enantioselectivity is still maintained. The synthetic utility was confirmed by transformations of the products. DFT calculations provide convincing evidence for the interpretation of stereoselection.
中文翻译:

酮亚胺和未活化二烯的催化不对称 Aza-Diels-Alder 反应
对映选择性氮杂-Diels-Alder反应可有效构建手性四氢吡啶,但酮亚胺与未活化二烯的催化不对称氮杂-Diels-Alder反应仍然是一个具有挑战性的课题。在此,在计算筛选的指导下,通过使用 B(C 6 F 5 ) 3实现了2-芳基-3 H-吲哚-3-酮与未活化二烯的高度对映选择性 aza-Diels-Alder 反应/手性磷酸催化剂体系,条件温和。该反应对于 aza-Diels-Alder 反应伙伴具有广泛的范围,因此可以快速获得一系列具有良好结果的四氢吡啶衍生物(产率高达 99%,>20:1 dr 和 98:2 er)。该反应非常有效:将模型反应的催化剂负载量降低到 0.1 mol%,仍然保持对映选择性。通过产品的转化证实了合成效用。DFT 计算为立体选择的解释提供了令人信服的证据。
更新日期:2021-07-27
中文翻译:

酮亚胺和未活化二烯的催化不对称 Aza-Diels-Alder 反应
对映选择性氮杂-Diels-Alder反应可有效构建手性四氢吡啶,但酮亚胺与未活化二烯的催化不对称氮杂-Diels-Alder反应仍然是一个具有挑战性的课题。在此,在计算筛选的指导下,通过使用 B(C 6 F 5 ) 3实现了2-芳基-3 H-吲哚-3-酮与未活化二烯的高度对映选择性 aza-Diels-Alder 反应/手性磷酸催化剂体系,条件温和。该反应对于 aza-Diels-Alder 反应伙伴具有广泛的范围,因此可以快速获得一系列具有良好结果的四氢吡啶衍生物(产率高达 99%,>20:1 dr 和 98:2 er)。该反应非常有效:将模型反应的催化剂负载量降低到 0.1 mol%,仍然保持对映选择性。通过产品的转化证实了合成效用。DFT 计算为立体选择的解释提供了令人信服的证据。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号