当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Regulation on Solvation Shell and Electrode Interface for Dendrite-Free Zn Ion Batteries Achieved by a Low-Cost Glucose Additive
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-25 , DOI: 10.1002/anie.202105756 Peng Sun 1 , Liang Ma 1 , Wanhai Zhou 2 , Meijia Qiu 1, 3 , Zilong Wang 1 , Dongliang Chao 2 , Wenjie Mai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-25 , DOI: 10.1002/anie.202105756 Peng Sun 1 , Liang Ma 1 , Wanhai Zhou 2 , Meijia Qiu 1, 3 , Zilong Wang 1 , Dongliang Chao 2 , Wenjie Mai 1
Affiliation
|
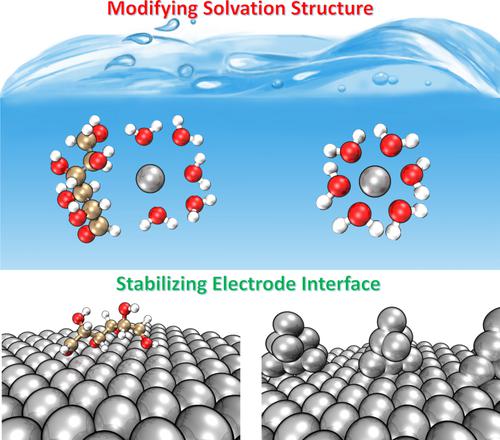
Dendrite growth and by-products in Zn metal aqueous batteries have impeded their development as promising energy storage devices. We utilize a low-cost additive, glucose, to modulate the typical ZnSO4 electrolyte system for improving reversible plating/stripping on Zn anode for high-performance Zn ion batteries (ZIBs). Combing experimental characterizations and theoretical calculations, we show that the glucose in ZnSO4 aqueous environment can simultaneously modulate solvation structure of Zn2+ and Zn anode-electrolyte interface. The electrolyte engineering can alternate one H2O molecule from the primary Zn2+-6H2O solvation shell and restraining side reactions due to the decomposition of active water. Concomitantly, glucose molecules are inclined to absorb on the surface of Zn anode, suppressing the random growth of Zn dendrite. As a proof of concept, a symmetric cell and Zn-MnO2 full cell with glucose electrolyte achieve boosted stability than that with pure ZnSO4 electrolyte.
中文翻译:

通过低成本葡萄糖添加剂实现无枝晶锌离子电池的溶剂化壳和电极界面的同时调节
锌金属水系电池中的枝晶生长和副产物阻碍了它们作为有前途的储能装置的发展。我们利用低成本添加剂葡萄糖来调节典型的 ZnSO 4电解质系统,以改善高性能锌离子电池 (ZIB) 锌负极上的可逆电镀/剥离。结合实验表征和理论计算,我们表明 ZnSO 4水性环境中的葡萄糖可以同时调节 Zn 2+和 Zn 阳极-电解质界面的溶剂化结构。电解质工程可以从初级 Zn 2+ -6H 2 中替代一个 H 2 O 分子由于活性水的分解,O 溶剂化壳和抑制副反应。同时,葡萄糖分子倾向于吸附在锌阳极表面,抑制了锌枝晶的随机生长。作为概念证明,对称电池和使用葡萄糖电解质的Zn-MnO 2全电池比使用纯 ZnSO 4电解质获得更高的稳定性。
更新日期:2021-08-03
中文翻译:

通过低成本葡萄糖添加剂实现无枝晶锌离子电池的溶剂化壳和电极界面的同时调节
锌金属水系电池中的枝晶生长和副产物阻碍了它们作为有前途的储能装置的发展。我们利用低成本添加剂葡萄糖来调节典型的 ZnSO 4电解质系统,以改善高性能锌离子电池 (ZIB) 锌负极上的可逆电镀/剥离。结合实验表征和理论计算,我们表明 ZnSO 4水性环境中的葡萄糖可以同时调节 Zn 2+和 Zn 阳极-电解质界面的溶剂化结构。电解质工程可以从初级 Zn 2+ -6H 2 中替代一个 H 2 O 分子由于活性水的分解,O 溶剂化壳和抑制副反应。同时,葡萄糖分子倾向于吸附在锌阳极表面,抑制了锌枝晶的随机生长。作为概念证明,对称电池和使用葡萄糖电解质的Zn-MnO 2全电池比使用纯 ZnSO 4电解质获得更高的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号