当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Strecker Reaction of Isatin-Derived N-Unsubstituted Ketimines
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-24 , DOI: 10.1021/acs.orglett.1c01194 Tetsuya Kadota 1 , Masanao Sawa 1 , Yuta Kondo 1 , Hiroyuki Morimoto 1 , Takashi Ohshima 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-24 , DOI: 10.1021/acs.orglett.1c01194 Tetsuya Kadota 1 , Masanao Sawa 1 , Yuta Kondo 1 , Hiroyuki Morimoto 1 , Takashi Ohshima 1
Affiliation
|
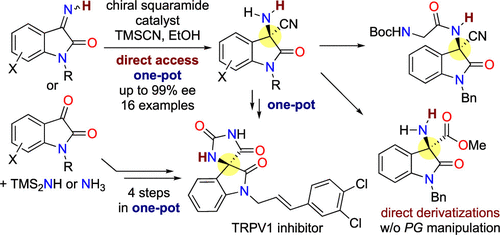
A catalytic enantioselective Strecker reaction of isatin-derived N-unsubstituted ketimines directly afforded the N-unprotected α-aminonitriles with a tetrasubstituted carbon stereocenter in up to 99% ee without requiring protection/deprotection steps. One-pot Strecker reactions from the parent carbonyl compounds were also realized with comparable yields and enantioselectivities. Direct transformations of the N-unprotected α-aminonitrile products streamlined the synthesis of unnatural amino acid derivatives and achieved the shortest one-pot stereoselective routes to a biologically active compound reported to date.
中文翻译:

靛红衍生的 N-未取代酮亚胺的催化对映选择性 Strecker 反应
靛红衍生的 N-未取代酮亚胺的催化对映选择性 Strecker 反应直接以高达 99% 的ee 提供具有四取代碳立体中心的 N-未保护的 α-氨基腈,而无需保护/脱保护步骤。母体羰基化合物的一锅 Strecker 反应也以相当的产率和对映选择性实现。N-未保护的 α-氨基腈产物的直接转化简化了非天然氨基酸衍生物的合成,并实现了迄今为止报道的最短的生物活性化合物的一锅立体选择性路线。
更新日期:2021-06-18
中文翻译:

靛红衍生的 N-未取代酮亚胺的催化对映选择性 Strecker 反应
靛红衍生的 N-未取代酮亚胺的催化对映选择性 Strecker 反应直接以高达 99% 的ee 提供具有四取代碳立体中心的 N-未保护的 α-氨基腈,而无需保护/脱保护步骤。母体羰基化合物的一锅 Strecker 反应也以相当的产率和对映选择性实现。N-未保护的 α-氨基腈产物的直接转化简化了非天然氨基酸衍生物的合成,并实现了迄今为止报道的最短的生物活性化合物的一锅立体选择性路线。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号