Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrostatic Reaction Inhibition in Nanoparticle Catalysis
Langmuir ( IF 3.7 ) Pub Date : 2021-05-25 , DOI: 10.1021/acs.langmuir.1c00903 Yi-Chen Lin 1 , Rafael Roa 2 , Joachim Dzubiella 1, 3
Langmuir ( IF 3.7 ) Pub Date : 2021-05-25 , DOI: 10.1021/acs.langmuir.1c00903 Yi-Chen Lin 1 , Rafael Roa 2 , Joachim Dzubiella 1, 3
Affiliation
|
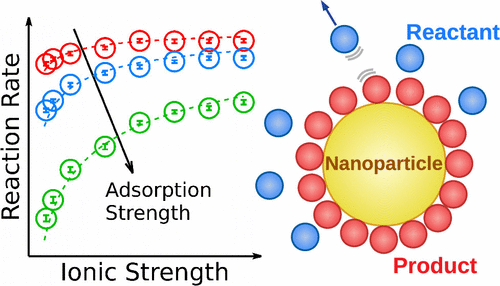
Electrostatic reaction inhibition in heterogeneous catalysis emerges if charged reactants and products with similar charges are adsorbed on the catalyst and thus repel the approaching reactants. In this work, we study the effects of electrostatic inhibition on the reaction rate of unimolecular reactions catalyzed on the surface of a spherical model nanoparticle using particle-based reaction-diffusion simulations. Moreover, we derive closed rate equations based on an approximate Debye–Smoluchowski rate theory, valid for diffusion-controlled reactions, and a modified Langmuir adsorption isotherm, relevant for reaction-controlled reactions, to account for electrostatic inhibition in the Debye–Hückel limit. We study the kinetics of reactions ranging from low to high adsorptions on the nanoparticle surface and from the surface- to diffusion-controlled limits for charge valencies 1 and 2. In the diffusion-controlled limit, electrostatic inhibition drastically slows down the reactions for strong adsorption and low ionic concentration, which is well described by our theory. In particular, the rate decreases with adsorption affinity because, in this case, the inhibiting products are generated at a high rate. In the (slow) reaction-controlled limit, the effect of electrostatic inhibition is much weaker, as semiquantitatively reproduced by our electrostatic-modified Langmuir theory. We finally propose and verify a simple interpolation formula that describes electrostatic inhibition for all reaction speeds (“diffusion-influenced” reactions) in general.
中文翻译:

纳米粒子催化中的静电反应抑制
如果带电的反应物和具有相似电荷的产物吸附在催化剂上,从而排斥接近的反应物,则多相催化中的静电反应抑制就会出现。在这项工作中,我们使用基于粒子的反应扩散模拟研究静电抑制对球形模型纳米粒子表面催化的单分子反应的反应速率的影响。此外,我们基于近似的 Debye-Smoluchowski 速率理论推导出闭合速率方程,适用于扩散控制反应,以及与反应控制反应相关的修正朗缪尔吸附等温线,以解释 Debye-Hückel 极限中的静电抑制。我们研究了从纳米颗粒表面从低吸附到高吸附的反应动力学,以及从表面到扩散控制的电荷价数 1 和 2 的限制。在扩散控制的限制中,静电抑制大大减慢了强吸附的反应和低离子浓度,我们的理论很好地描述了这一点。特别是,速率随着吸附亲和力而降低,因为在这种情况下,抑制产物以高速率产生。在(慢)反应控制极限下,静电抑制的影响要弱得多,正如我们的静电修正朗缪尔理论半定量再现的那样。我们最终提出并验证了一个简单的插值公式,该公式通常描述了所有反应速度(“扩散影响”反应)的静电抑制。
更新日期:2021-06-08
中文翻译:

纳米粒子催化中的静电反应抑制
如果带电的反应物和具有相似电荷的产物吸附在催化剂上,从而排斥接近的反应物,则多相催化中的静电反应抑制就会出现。在这项工作中,我们使用基于粒子的反应扩散模拟研究静电抑制对球形模型纳米粒子表面催化的单分子反应的反应速率的影响。此外,我们基于近似的 Debye-Smoluchowski 速率理论推导出闭合速率方程,适用于扩散控制反应,以及与反应控制反应相关的修正朗缪尔吸附等温线,以解释 Debye-Hückel 极限中的静电抑制。我们研究了从纳米颗粒表面从低吸附到高吸附的反应动力学,以及从表面到扩散控制的电荷价数 1 和 2 的限制。在扩散控制的限制中,静电抑制大大减慢了强吸附的反应和低离子浓度,我们的理论很好地描述了这一点。特别是,速率随着吸附亲和力而降低,因为在这种情况下,抑制产物以高速率产生。在(慢)反应控制极限下,静电抑制的影响要弱得多,正如我们的静电修正朗缪尔理论半定量再现的那样。我们最终提出并验证了一个简单的插值公式,该公式通常描述了所有反应速度(“扩散影响”反应)的静电抑制。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号