当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Fenton Reaction in Water Assisted by Picolinic Acid: Accelerated Iron Cycling and Co-generation of a Selective Fe-Based Oxidant
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-05-25 , DOI: 10.1021/acs.est.1c00230 Zhichao Yang 1, 2 , Chao Shan 1, 3 , Bingcai Pan 1, 3 , Joseph J Pignatello 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-05-25 , DOI: 10.1021/acs.est.1c00230 Zhichao Yang 1, 2 , Chao Shan 1, 3 , Bingcai Pan 1, 3 , Joseph J Pignatello 2
Affiliation
|
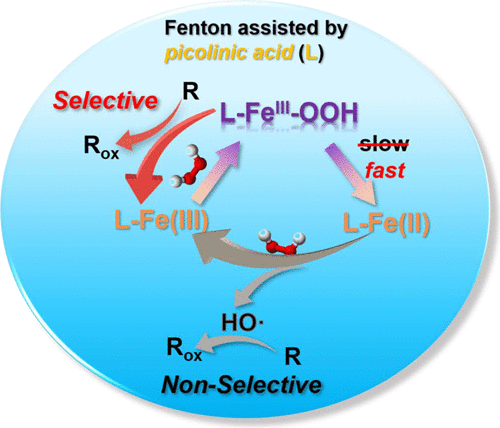
The Fenton reaction is limited by a narrow acidic pH range, the slow reduction of Fe(III), and susceptibility of the nonselective hydroxyl radical (HO•) to scavenging by water constituents. Here, we employed the biodegradable chelating agent picolinic acid (PICA) to address these concerns. Compared to the classical Fenton reaction at pH 3.0, PICA greatly accelerated the degradation of atrazine, sulfamethazine, and various substituted phenols at pH 5.0 in a reaction with autocatalytic characteristics. Although HO• served as the principal oxidant, a high-spin, end-on hydroperoxo intermediate, tentatively identified as PICA–FeIII–OOH, also exhibited reactivity toward several test compounds. Chloride release from the oxidation of 2,4,6-trichlorophenol and the positive slope of the Hammett correlation for a series of halogenated phenols were consistent with PICA–FeIII–OOH reacting as a nucleophilic oxidant. Compared to HO•, PICA–FeIII–OOH is less sensitive to potential scavengers in environmental water samples. Kinetic analysis reveals that PICA facilitates Fe(III)/Fe(II) transformation by accelerating Fe(III) reduction by H2O2. Autocatalysis is ascribed to the buildup of Fe(II) from the reduction of Fe(III) by H2O2 as well as PICA oxidation products. PICA assistance in the Fenton reaction may be beneficial to wastewater treatment because it favors iron cycling, extends the pH range, and balances oxidation universality with selectivity.
中文翻译:

吡啶甲酸辅助水中的芬顿反应:加速铁循环和选择性铁基氧化剂的联产
Fenton 反应受限于狭窄的酸性 pH 范围、Fe(III) 的缓慢还原以及非选择性羟基自由基 (H2O • ) 对水成分清除的敏感性。在这里,我们采用可生物降解的螯合剂吡啶甲酸 (PICA) 来解决这些问题。与 pH 3.0 下的经典芬顿反应相比,PICA 在具有自催化特性的反应中,在 pH 5.0 下大大加速了莠去津、磺胺二甲嘧啶和各种取代酚的降解。尽管 H2O •作为主要氧化剂,但一种高自旋、末端过氧化氢中间体,初步鉴定为 PICA-Fe III-OOH,也表现出对几种测试化合物的反应性。2,4,6-三氯苯酚氧化释放的氯化物和一系列卤代苯酚的哈米特相关性的正斜率与 PICA-Fe III - OOH 作为亲核氧化剂反应一致。与 H2O •相比,PICA–Fe III –OOH 对环境水样中的潜在清除剂不太敏感。动力学分析表明,PICA 通过加速 H 2 O 2 对Fe(III) 的还原来促进 Fe(III)/Fe(II) 转变。自催化归因于通过 H 2 O 2还原 Fe(III) 生成 Fe(II)以及 PICA 氧化产物。Fenton 反应中的 PICA 辅助可能有利于废水处理,因为它有利于铁循环,扩大 pH 范围,并平衡氧化普遍性和选择性。
更新日期:2021-06-15
中文翻译:

吡啶甲酸辅助水中的芬顿反应:加速铁循环和选择性铁基氧化剂的联产
Fenton 反应受限于狭窄的酸性 pH 范围、Fe(III) 的缓慢还原以及非选择性羟基自由基 (H2O • ) 对水成分清除的敏感性。在这里,我们采用可生物降解的螯合剂吡啶甲酸 (PICA) 来解决这些问题。与 pH 3.0 下的经典芬顿反应相比,PICA 在具有自催化特性的反应中,在 pH 5.0 下大大加速了莠去津、磺胺二甲嘧啶和各种取代酚的降解。尽管 H2O •作为主要氧化剂,但一种高自旋、末端过氧化氢中间体,初步鉴定为 PICA-Fe III-OOH,也表现出对几种测试化合物的反应性。2,4,6-三氯苯酚氧化释放的氯化物和一系列卤代苯酚的哈米特相关性的正斜率与 PICA-Fe III - OOH 作为亲核氧化剂反应一致。与 H2O •相比,PICA–Fe III –OOH 对环境水样中的潜在清除剂不太敏感。动力学分析表明,PICA 通过加速 H 2 O 2 对Fe(III) 的还原来促进 Fe(III)/Fe(II) 转变。自催化归因于通过 H 2 O 2还原 Fe(III) 生成 Fe(II)以及 PICA 氧化产物。Fenton 反应中的 PICA 辅助可能有利于废水处理,因为它有利于铁循环,扩大 pH 范围,并平衡氧化普遍性和选择性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号