Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-05-25 , DOI: 10.1016/j.tetlet.2021.153209 Shaoqing Du , Jin Li , Wen Fu , Xiaoyong Xu , Xusheng Shao , Xuhong Qian
|
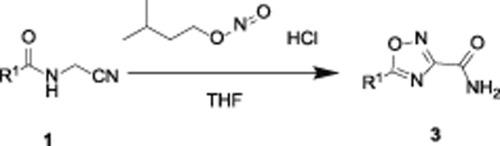
1,2,4-Oxadiazole-3-carboxamide has been extensively used in the pharmaceutical chemistry. In this study, 1,2,4-oxadiazole-3-carboxamide is accomplished through an acid-promoted reaction of N-(cyanomethyl)amide with nitrosation reagent. This novel preparation of 1,2,4-oxadiazole-3-carboxamide was carried out at 25 °C, and the yield of target compounds was as high as 92%. At the same time, the amount of acid used is reduced. A mechanism speculation for the formation of 1,2,4-oxadiazole-3-carboxamide has been provided. The new synthetic method provides great convenience for the synthesis of compounds containing 1,2,4-oxadiazole-3-carboxamide.
中文翻译:

N- (氰甲基)酰胺与亚硝化试剂的酸促进反应:1,2,4-恶二唑-3-甲酰胺的简易合成
1,2,4-Oxadiazole-3-carboxamide 已广泛用于药物化学。在这项研究中,1,2,4-oxadiazole-3-carboxamide 是通过N-(氰甲基)酰胺与亚硝化试剂的酸促进反应来完成的。这种1,2,4-恶二唑-3-甲酰胺的新制备在25℃下进行,目标化合物的产率高达92%。同时,减少了酸的用量。已经提供了形成 1,2,4-oxadiazole-3-carboxamide 的机制推测。新的合成方法为含1,2,4-恶二唑-3-甲酰胺化合物的合成提供了极大的便利。






























 京公网安备 11010802027423号
京公网安备 11010802027423号